Initiative will transform public understanding and conversation about dental tech; updated Carestream Dental branding reinforces digital focus
Carestream Dental, an industry-leading provider of integrated digital dental solutions, introduced its Digital Dentistry Difference public education campaign today at the Chicago Dental Society Midwinter Meeting. Through this campaign, the company will directly educate consumers about the benefits of digital dentistry and will arm doctors with resources to prompt conversation with their patients, solving a significant awareness gap that research suggests is harming patient perception of doctors and the oral healthcare industry overall.
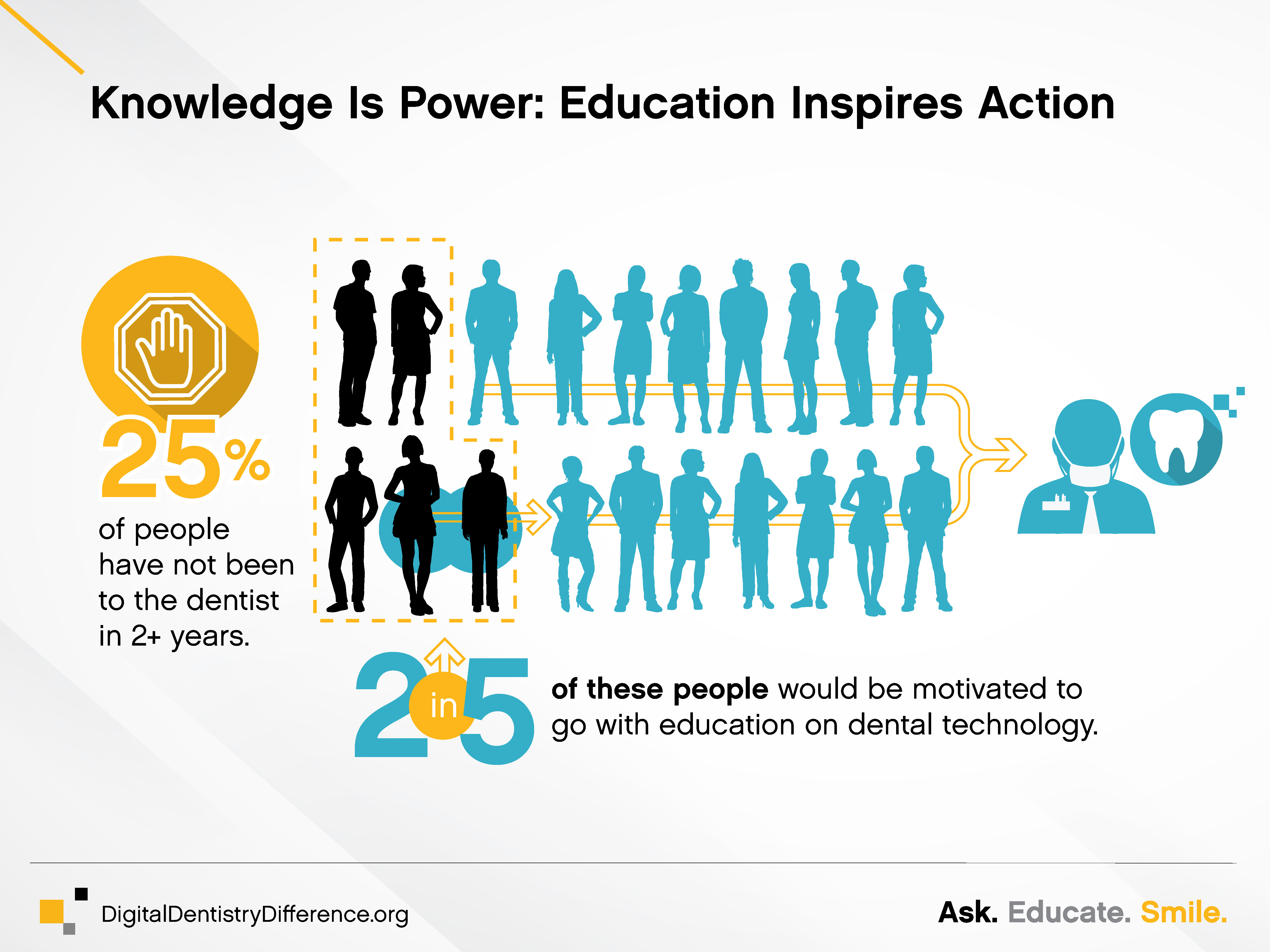
According to the first Global Digital Dentistry survey—commissioned by Carestream Dental in November 2019 to measure public attitudes, perceptions and behaviors related to dental technology—patients across the globe believe that the oral healthcare industry lags behind both optical and orthopedic in terms of the technology used. Even more surprising, less than half of patients even believe their doctor uses very advanced technology.
“It is a missed opportunity when you consider that doctors are investing in digital dentistry to improve patient care, yet many patients do not understand the level of advanced digital technology the industry offers, or how it can change their lives,” Ed Shellard, D.M.D., chief dental officer, Carestream Dental. “The Digital Dentistry Difference campaign will change that by speaking directly to consumers about why they should be asking about the technology used by their doctor, and at the same time, arming doctors with the tools they need to easily educate. We’re proud to be leading the charge with this campaign and look forward to the industry working together to create more smiles all around.”
As part of the Digital Dentistry Difference campaign, Carestream Dental will be directly reaching patients through a large-scale public relations initiative, by connecting digital dentistry experts with national and regional news media to share information on how the technology truly makes a difference. As part of these ongoing efforts, Carestream Dental will create a groundswell of conversation about digital technology ahead of Oral Health Month. The company is partnering with Dr. Miguel Stanley to conduct a broadcast media tour in late May, with plans to reach regional markets throughout the U.S. and encourage viewers to talk to their doctors about the digital technology used in their care.
Not only will public education about digital dentistry increase patient awareness, it is also likely to benefit doctors, according to Carestream Dental’s survey results. The survey revealed that that education is likely to help:
• Increase patient retention: Two out of three patients would consider changing to a dentist who uses more advanced technology.
• Bring in new patients: Approximately two in five patients who haven’t been to the dentist in two or more years say they would be motivated to go with education about how technology would improve their care.
• Enhance patient perception of care: 95 percent of patients who experienced advanced technology agree they get better care.
The Digital Dentistry Difference: Global Consumer Survey results have been published in a white paper, which can be found at DigitalDentistryDifference.org, along with a suite of complimentary educational resources doctors can download and use in their own patient education efforts. The tools available include the Digital Dentistry Difference video, a series of infographics and patient education materials, which can be shared through online and social channels, shared in a doctor’s waiting room or referenced during patient consultations.
Bringing Digital Dentistry to Life
Carestream Dental’s commitment to digital is engrained in the business’ core. In addition to its new education campaign, the company has also launched a refreshed brand look to further demonstrate its dedication to digital.
“We’re already walking the walk with our product offerings, and now it’s time to talk the talk in the way we communicate about them,” Greg Marko, chief marketing officer, Carestream Dental, said. “This brand refresh fully embraces the spirit of Carestream Dental—who we are today and where we, and the industry as a whole, are headed. Digital is at the heart of everything we do, and our refreshed brand now reinforces that even more clearly.”
The new Carestream Dental branding can be experienced firsthand in booth #3602 during the Midwinter Meeting exhibition, Feb. 20-22.
For more information on Carestream Dental and its products, visit carestreamdental.com.




 Glidewell today unveiled a new esthetic formulation of its popular
Glidewell today unveiled a new esthetic formulation of its popular 


 DenMat Holdings, LLC (“DenMat”), announces that it is entering the clear aligner market after receiving its FDA 510k for its new system in December of 2019. The 510k clearance allows the company to market, sell, design, manufacture and deliver orthodontic clear aligners to dental professionals and dental laboratories alike. Recently, the company acquired rights to the tradename “OrthoClear”, which will serve as the flagship brand for this new service offering.
DenMat Holdings, LLC (“DenMat”), announces that it is entering the clear aligner market after receiving its FDA 510k for its new system in December of 2019. The 510k clearance allows the company to market, sell, design, manufacture and deliver orthodontic clear aligners to dental professionals and dental laboratories alike. Recently, the company acquired rights to the tradename “OrthoClear”, which will serve as the flagship brand for this new service offering.


