Is a less painful dental injection worth more money? The vast majority of dental patients think so. More than 7 out of 10 patients said they’d pay extra if their dentist used a faster, less painful local anesthetic, according to a new survey from dental pharmaceutical company Balanced pHarma.
Balanced pHarma published the results of its online survey on July 28 that asked dozens of dental patients about their experiences with dental procedures and whether they’d be willing to shell out extra cash for less painful injections.
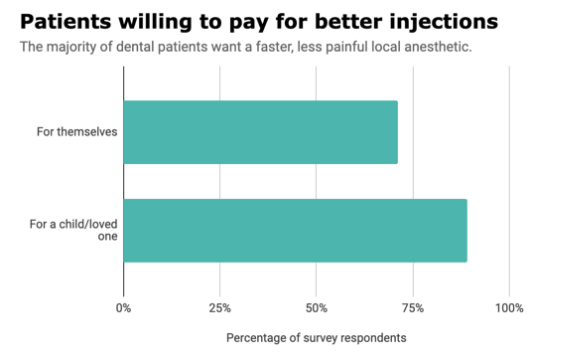
More than 7 out of 10 dental patients said they’d be willing to pay extra if their dentist used a faster, less painful anesthetic, and more than half were willing to pay at least 10% more for the procedure.
Not surprisingly, respondents were even more concerned about injection pain when asked about a child or loved one, with 9 out of 10 patients willing to pay extra and three-fourths willing to pay at least 10% more for the procedure.
”The survey responses were a wonderful reminder of just how much people care about others,” said Dr. Scott Keadle, Chief Medical Officer at Balanced pHarma. “Clearly, people want dental injections to be less painful, but they really want their loved ones to have less painful injections.”
A majority of patients reported that painful injections were the thing they feared most about a dental visit. This could be why more than one-third said they would switch dentists to find one that offered a less painful anesthetic option.
“These survey findings show that dental practices might be able to help patients and save time by offering a buffered local anesthetic,” Keadle added.
Other survey findings:
● Fear of injections kept 12% of patients from visiting the dentist as often as they should.
● A painless injection was a “must-have when selecting a dentist” for 47% of respondents.
Buffered anesthetics cause less pain
The survey findings make it clear that a large majority of dental patients want a less painful dental injection, and buffered anesthetics offer one way to achieve this.
Research shows that neutralizing the acidity of local anesthetics (buffering) makes the pH-balanced drugs less toxic1, less painful2, faster acting3, and more likely to achieve successful anesthesia4 than conventional injections.
Buffered anesthetics improve productivity
With quicker onset times and more reliable anesthesia, pH-balanced anesthetics also offer more efficiency for dental service providers. Balanced Pharma crunched the numbers and found practices could raise productivity by 12%.
“pH-balanced anesthetics not only help patients survive the injection, but they also give clinicians a faster-acting and more reliable drug that increases productivity,” Keadle said.
About Balanced pHarma and Libracaine
Balanced pHarma, with headquarters in Cornelius, North Carolina, is a medical and dental pharmaceutical company that develops better local anesthetic drugs to improve patient comfort and safety.
Libracaine is a new pH-balanced (buffered) anesthetic that doesn’t cause pain on injection and works much faster than traditional anesthetics. Unlike other buffered anesthetic products, Libracaine will be offered in a standard dental cartridge that can be used in a standard dental syringe. It will also cost about two thirds less than the current buffered products.
Libracaine is currently in development and not approved by the U.S. Food and Drug Administration for sale or distribution.
Learn more at BalancedPharma.com.
About the Survey
In May 2020, Balanced pHarma conducted an informal online survey of 92 dental patients. The survey asked participants about their dental office experiences, including what they found most fearful about visiting the dentist and how they feel about dental injections.
References:
1. Warren, et al, J Oral Maxillofac Surg 75:1363-1366, 2017
2. Guo, et al, J Dent Anesth Pain Med 2018;18(3):129-142
3. Malamed, Falkel, et al, Compendium, Feb 2013; Vol. 34:1, pp. 10-20
4. Kattan, et al, JADA 2019:150(3):165-177






 Sonendo®, Inc., a leading dental technology company and developer of the GentleWave® System, today announced that the GentleWave System creates virtually no aerosols during root canal treatment.
Sonendo®, Inc., a leading dental technology company and developer of the GentleWave® System, today announced that the GentleWave System creates virtually no aerosols during root canal treatment.


 Arfona, a leader in 3D printing and digital technology for dental applications, has announced the opening of its state-of-the-art 3D printing service bureau. The 3D manufacturing facility and testing laboratory is part of a joint venture between Arfona, LLC, and Valplast International Corp. and operates under the name Arfona Printing Services. Funding for the venture was provided by RFC Capital Partners Fund 3, LLC.
Arfona, a leader in 3D printing and digital technology for dental applications, has announced the opening of its state-of-the-art 3D printing service bureau. The 3D manufacturing facility and testing laboratory is part of a joint venture between Arfona, LLC, and Valplast International Corp. and operates under the name Arfona Printing Services. Funding for the venture was provided by RFC Capital Partners Fund 3, LLC. Taking into account all the different factors for its customers and employees and following intensive discussions, Dentsply Sirona has decided the Company will not attend the International Dental Show (IDS), scheduled to be held from March 9th to 13th, 2021 in Cologne, Germany. This also applies to Dentsply Sirona brands VDW, MIS and Zhermack.
Taking into account all the different factors for its customers and employees and following intensive discussions, Dentsply Sirona has decided the Company will not attend the International Dental Show (IDS), scheduled to be held from March 9th to 13th, 2021 in Cologne, Germany. This also applies to Dentsply Sirona brands VDW, MIS and Zhermack. 2C MedTech, a healthcare product developer and distributor specializing in commercializing real innovations with true impact, is partnering with CASPR™ Group to introduce CASPR’s next generation disinfection technology to dental offices across the globe.
2C MedTech, a healthcare product developer and distributor specializing in commercializing real innovations with true impact, is partnering with CASPR™ Group to introduce CASPR’s next generation disinfection technology to dental offices across the globe.
