Treatment of a GRD With a Hydrated ADM
Clinical and histologic evaluation demonstrate graft incorporation and stability
Douglas H. Mahn, DDS | Duane R. Schafer, DDS, MS
Using an acellular dermal matrix (ADM) instead of a connective tissue graft to treat a gingival recession defect (GRD) eliminates the limitations posed by tissue availability and donor site morbidity. Although the treatment of GRDs using ADMs is well-documented,1-3 there are few articles that describe the histologic findings of these treatments. In 1998, Harris evaluated punch biopsies taken from sites that were treated with a freeze-dried ADM material.1 He found that the graft was incorporated into the tissue instead of being absorbed or exfoliated. In 2002, Richardson and Maynard published a histologic case report of a GRD site that was treated using a freeze-dried ADM4; however, in this case, a significant reduction in the size of the graft matrix was found. In 2005, Cummings and colleagues performed block section biopsies of sites treated with freeze-dried ADMs, and their histologic findings were similar to those described in Harris's report.5 In all three of these studies, when the freeze-dried ADM was placed, it was completely covered by the mucogingival flap.
Recently, a hydrated ADM material became available for use in periodontal surgery. The following case report describes the clinical and histologic results of treating a Cairo recession type 1 (RT1) GRD using a hydrated ADM.
Case Report
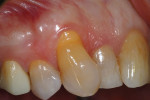
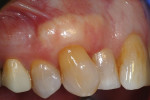
A 66-year-old, nonsmoking female patient presented for a periodontal evaluation. Her overall periodontal status was healthy. Her primary concern was a progressively deteriorating GRD that was affecting her maxillary right canine (tooth No. 6) (Figure 1). During the clinical examination, intraoral measurements were made using a periodontal probe (UNC-15, Hu-Friedy) and rounded to the nearest millimeter. The buccal surface of tooth No. 6 was found to have a 3-mm RT1 GRD and a noncarious lesion. In addition, the straight facial periodontal probing depth was determined to be 3 mm, and the width of the attached keratinized gingiva was determined to be 2 mm. It was also noted that tooth No. 6 was crowded, with its mesial aspect buccal to tooth No. 7.
Treatment
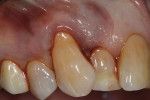
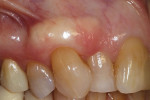
After treatment options were presented, the patient elected to proceed with a surgical intervention involving a hydrated ADM material (AlloDerm SELECT™ RTM, BioHorizons). Local anesthesia was achieved using 2% lidocaine with 1:100,000 epinephrine, and then any root surface irregularities were reduced using sharp hand instruments. Next, intrasulcular incisions were made on the buccal aspects of teeth Nos. 5 through 7 with a scalpel (No. 15 scalpel blade, Bard-Parker) (Figure 2). Care was taken to not traumatize the interdental papillae. Using an Orban knife, a full thickness flap was elevated beneath the attached keratinized gingiva, and then a split thickness flap was elevated below the mucosal tissues.
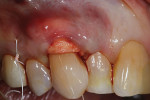
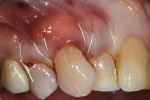
The hydrated ADM material, which had a thickness of approximately 1.6 mm, was trimmed to be approximately 15 mm × 5 mm (Figure 3), inserted underneath the mucogingival flap, and positioned to lay evenly on either side of the root. The basement side of the membrane was positioned toward the root. Next, beginning on the facial gingiva distal to tooth No. 5, a continuous 4.0 monofilament non-absorbable polytetrafluoroethylene (PTFE) suture (Cytoplast™ Non-Absorbable PTFE Sutures, Osteogenics) was weaved around from the lingual aspect to the buccal aspect of the tooth to secure the ADM into position (Figure 4). The flap was then coronally advanced to completely cover the ADM, and tension-free coronal advancement was confirmed. Using the same suture, the flap was secured in place by weaving the suture to the mesial aspect of tooth No. 7 and then in the reverse direction until the final knot was tied over the original knot (Figure 5).
Postoperative Care and Follow-Up
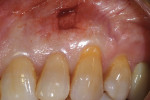
Postoperative instructions were given to the patient, which included not brushing or flossing the surgical site for 7 days. Instead, she was instructed to rinse twice daily with a 0.12% chlorhexidine gluconate solution (Peridex™, 3M). A 10-day regimen of amoxicillin (875 mg q12h) was prescribed, and ibuprofen (600 mg) was prescribed for discomfort. After 7 days, the patient returned to the office and the sutures were removed. She was instructed to discontinue using the rinse and to begin gentle tooth brushing and flossing. After 6 weeks, the patient returned so that the progress of the healing could be evaluated (Figure 6). The tooth No. 6 site was found clinically to have healed well, and it demonstrated complete root coverage. With the light appearance and distinct border of the implanted hydrated ADM, its presence and dimensions were evident. Normal tooth brushing and flossing was reinstated at this time.
Histologic Evaluation
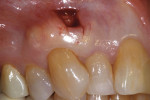
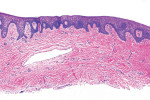
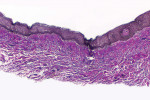
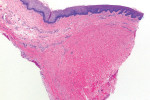
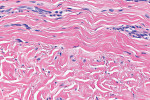
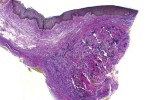
After 19 weeks of healing, the patient returned for an investigational biopsy of the graft site. At this time, the tooth No. 6 site continued to demonstrate complete root coverage. Beneath the mucogingival tissues, the outline of the implanted hydrated ADM was distinct, and there was no apparent reduction in its dimensions (Figure 7). Written consent was obtained from the patient. To obtain the biopsy, a No. 15 scalpel blade was used to make incisions that were approximately 4 mm in length at the mesial-apical edge of the implanted hydrated ADM. These incisions were made to the depth of the underlying bone (Figure 8). For comparison purposes, a similar biopsy was acquired from the untreated contralateral site mesial to tooth No. 11 (Figure 9). The biopsy from the grafted site appeared to be thicker than the one from the ungrafted site. A histologic evaluation of both biopsies was performed using hematoxylin and eosin (H&E) stain and Verhoeff's stain.
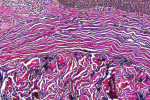
The histologic evaluation showed collagen that aligned horizontally, in a mostly parallel nature, to the superficial epithelium in both the ungrafted (Figure 10 and Figure 11) and grafted (Figure 12 through Figure 15) biopsy sites. In the grafted specimens, a clear demarcation at the interface of the native tissue and the implanted tissue was present. The implanted tissue, which was characterized by dense bands of disorganized collagen matrix, appeared well incorporated and without inflammatory cells. The Verhoeff's stain demonstrated the presence of numerous darkly stained elastin fibers in the implanted tissue that were inconspicuous in the native tissue.
Discussion
Properly processed ADMs are immunologically inert. During processing, the native structural and biochemical components of ADMs are preserved to maintain their biologic and mechanical properties6; however, the decellularization process removes all the cells and cellular debris to prevent a foreign body response by the host.7,8 Because cellular antigens are recognized by the host and can result in an adverse inflammatory response or immune-mediated rejection,9,10 effective decellularization is critical to the success of the biologic scaffold.11
The hydrated ADM used in this case was processed using a patented technique and stored in a patented aqueous phosphate-buffered preservation solution.12 In addition, it underwent terminal sterilization that included electron beam irradiation to meet a sterility assurance level of 10-3.12 Terminal sterilization is a validated process in which tissue is sterilized within its final barrier system (ie, package, container).13,14
The H&E and Verhoeff's stains allowed the collagen architecture of the native tissue to be clearly distinguished from that of the implanted tissue. The superficial native tissue exhibited collagen fibers that aligned parallel to each other and the epithelium, whereas the implanted tissue exhibited collagen fibers that appeared disordered. The implanted tissue, however, was markedly thicker than the overlying native tissue. Verhoeff's stain, which stains elastin fibers, has been used to identify implanted ADMs in grafted GRD sites.1,4,15 In this case, the abundant darkly stained elastin fibers in the grafted specimen indicate that the hydrated ADM was incorporated and not absorbed or exfoliated. The absence of observable inflammatory cells indicated that the implanted hydrated ADM was immunologically inert.
The complete root coverage and visible presence of the implanted graft at 19 weeks provided clinical evidence of the success of the treatment, and the results of the histologic analysis showed the incorporation of the hydrated ADM into the surrounding tissues. These short-term findings support the use of a hydrated ADM with a coronally advanced flap to treat RT1 GRDs; however additional investigations are needed to evaluate the long-term success of this intervention.
About the Author
Douglas H. Mahn, DDS
Private Practice
Manassas, Virginia
Duane R. Schafer, DDS, MS
Professor
Chair, Endodontics and Oral Diagnostic Sciences
Interim Chair, Pediatric Dentistry
School of Dentistry
Virginia Commonwealth University
Richmond, Virginia
References
1. Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: a clinical and histological evaluation of a case report. J Periodontol. 1998;69(11):1305-1311.
2. Henderson RD, Greenwell H, Drisko C, et al. Predictable multiple site root coverage using an acellular dermal matrix allograft. J Periodontol. 2001;72(5):571-582.
3. Mahn DH. Treatment of gingival recession with a modified "tunnel" technique and an acellular dermal connective tissue allograft. Pract Proc Aesthetic Dent. 2001;13(1):69-74.
4. Richardson CR, Maynard JG. Acellular dermal graft: a human histologic case report. Int J Periodontics Restorative Dent. 2002;22(1):21-29.
5. Cummings LC, Kaldahl WB, Allen EP. Histologic evaluation of autogenous connective tissue and acellular dermal matrix grafts in humans. J Periodontol. 2005;76(2):178-86.
6. Harper JR, McQuillan DJ. Extracellular wound matrices: a novel regenerative tissue matrix (RTM) technology for connective tissue reconstruction. Wounds. 2007;19(6):163-168.
7. Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71(2):343-354.
8. Livesey SA, Herndon DN, Hollyoak MA, et al. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60(1):1-9.
9. Erdag G, Morgan JR. Allogeneic versus xenogeneic immune reaction to bioengineered skin grafts. Cell Transplant. 2004;13(6):701-712.
10. Gock H, Murray-Segal L, Salvaris E, et al. Allogeneic sensitization is more effective than xenogeneic sensitization in eliciting Gal-mediated skin graft rejection. Transplantation. 2004;77(5):751-753.
11. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5(1):1-13.
12. AlloDerm Select™ and AlloDerm Select GBR™ Regenerative Tissue Matrix instructions for use, 2021.
13. American Association of Tissue Banks. Guidance document: microbiologic process validation & surveillance program [No. 5, version 2, July 18, 2016]. AATB website. https://www.aatb.org/sites/default/files/guidance-docs/AATB-Guidance-Document-No5-v2-07-18-16.pdf. Published July 18, 2016. Accessed October 14, 2022.
14. US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: sterile drug products manufactured by aseptic processing - current good manufacturing practice. FDA website. https://www.fda.gov/media/71026/download. Published September 2004. Accessed October 14, 2022.
15. Kazlouskaya V, Malhotra S, Lambe J, et al. The utility of elastic Verhoeff-Van Gieson staining in dermatopathology. J Cutan Pathol. 2013;40(2):211-225.