Treatment of Peri-Implantitis in the Esthetic Zone
Adjunctive dermal allograft thickens soft tissue, may reduce mucosal recession
Barry P. Levin, DMD
Peri-implantitis has been classified as an inflammatory disease, resulting in the loss of previously osseointegrated bone. This is to be differentiated from peri-implant mucositis, which is characterized by inflammation within the mucosa that does not result in bone loss.1 Berglundh’s definition of peri-implantitis included the presence of probing depths greater than 6 mm or attachment loss or bone loss of at least 2.5 mm. Communication between clinicians, as well as between doctors and patients, regarding the identification and extent of peri-implant disease remains critical for the long-term maintenance of previously placed and restored implants.2 In 2012, Froum and Rosen proposed a classification system for peri-implantitis using the presence of bleeding and/or suppuration, probing depths, and the degree of bone loss as criteria. They classified this disease as early, moderate, or advanced based on probing depths of at least 4.0 mm, 6.0 mm, or 8.0 mm along with bone loss of less than 25%, 25% to 50%, or greater than 50%, respectively.3
Regardless of the extent of peri-implantitis when first diagnosed, the goals of therapy should be to arrest the disease process and, when appropriate, prolong the lifespan of the affected implant. Therapy may include non-surgical and/or surgical interventions.4,5,6,7 All surgical interventions are geared toward surface decontamination, using either a resective or regenerative approach. Froum and colleagues demonstrated that a combination of mechanical and serial chemical decontamination followed by bone augmentation can achieve stable results.8
The following case report demonstrates the surgical management of peri-implantitis involving an implant in the esthetic zone. Both mechanical and chemotherapeutic implant surface cleansing techniques were utilized prior to regenerative therapy. The goal of treatment was not only to arrest the disease process but also to regenerate the supporting bone structure that was lost and preserve esthetics.
Case Report
In 2007, a 47-year-old female patient underwent immediate extraction of tooth No. 8 followed by implant placement. The procedure included simultaneous bone grafting, guided bone regeneration, and immediate provisionalization. Approximately 3 months after surgery, the implant was definitively restored, and it remained asymptomatic and demonstrated probing depths of less than 4.0 mm for almost 8 years.
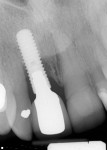
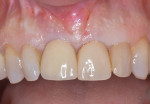
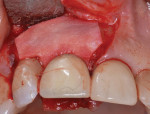
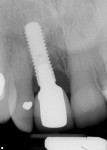
During a future appointment for routine maintenance, the site displayed edema, suppuration upon probing, and radiographic bone loss. The developing lesion also communicated with the mesial aspect of tooth No. 7, which exhibited a probing depth of 7.0 mm on the mesio-facial surface (Figure 1). Nonsurgical debridement was performed, antibiotics were prescribed (875 mg of Amoxicillin taken twice daily for 7 days), and she was scheduled to return approximately 4 weeks later for surgical therapy (Figure 2).
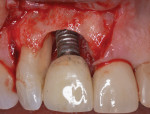
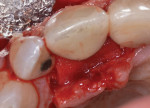
After the patient returned for surgery and was administered a local anesthetic (3.4 cc of 4% articaine with epinephrine 1/100,000 and 1.8 cc of 2% lidocaine with epinephrine 1/50,000), a facial trapezoidal flap was reflected from tooth No. 7 to the mesial aspect of the implant in the No. 8 position. Vertical releasing incisions were made to facilitate defect visualization and preserve the papilla between tooth No. 8 and tooth No. 9. Next, the defect was debrided with titanium curettes and titanium ultrasonic tips (Acteon). Using the same titanium ultrasonic tips, the implant surface was then thoroughly instrumented to remove all visible hard deposits and produce a visually clean surface. No effort was made to remove the rough surface via implantoplasty. The root of tooth No. 7 was instrumented using the ultrasonic tips and Gracey curettes (American Eagle Instruments). The implant was then burnished using a series of sterile gauze squares saturated with chlorhexidine, doxycycline, and sterile saline, respectively. Each was applied to the implant surfaces for about 1 minute. After burnishing, the root of tooth No. 7 was treated with doxycycline for about 3 minutes, followed by copious irrigation with a sterile saline solution (Figure 3).
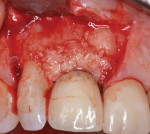
At the inception of surgery, recombinant human platelet-derived growth factor-BB (rhPDGF-BB) (Gem 21S, Osteohealth) was used to hydrate 0.5 g of mineralized bone allograft (FDBA; MTF) and a 1.0 cm x 2.0 mm strip of human dermal allograft (PerioDerm, Dentsply). The defects were obturated from the facial and palatal aspects with the growth factor enhanced bone allograft. To facilitate revascularization of the graft via the clot surrounding the particulate, over condensing of the particulate was avoided (Figure 4).
The enhanced dermal allograft was trimmed to fit over the facial and palatal aspects of the defects, overlapping the borders of the defects onto sound bone by about 2.0 mm to 3.0 mm (Figure 5 and Figure 6). The allograft was utilized for graft containment and barrier function around tooth No. 7 (ie, guided tissue regeneration) and the implant site of tooth No. 8 (ie, guided bone regeneration). The orientation of the allograft was intentional in that the basement membrane side was directed towards the bone graft and defect borders, while the connective tissue side, which absorbs blood, was oriented “outwards” to be in contact with the periosteum of the flap. This was done to promote integration of the allograft with the overlying soft tissues.
Primary closure was achieved with interrupted and sling resorbing (5-0 MONOCRYL, 5-0 VICRYL Rapide; Ethicon) sutures, without tension on the flap (Figure 7 and Figure 8).
The patient was prescribed amoxicillin (875 mg taken twice daily for 10 days); etodolac (400 mg taken three times per day for 3 days), a non-steroidal, anti-inflammatory medication that reduces postoperative swelling; and 0.12% chlorhexidine rinses (to be used twice daily for 30 seconds in lieu of manual brushing until sutures are removed or absorbed at 10 days).
The healing period was uneventful, and several sutures were easily removed at the 10-day postoperative appointment. Following this, the patient was instructed to brush with an extra-soft manual toothbrush using the “roll technique” and perform only supragingival flossing for the next 4 weeks. Probing by the dentist, periodontist, and multiple hygienists was avoided for 12 months.
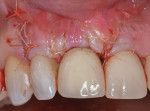
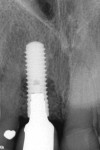
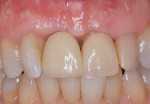
At approximately 14 months, during a routine periodontal maintenance appointment, clinical health was demonstrated (Figure 9). No probing depth greater than 4.0 mm was evident around the No. 8 implant, and tooth No. 7 could not be probed deeper than 3.0 mm on the medial aspect. Radiographically, suggestion of bone fill and re-osseointegration were evident (Figure 10). The vertical component of the peri-implant defect appeared to be resolved with several coronal threads in a supracrestal location. There was also evidence of periodontal attachment gain with reconstitution of a periodontal ligament space on the mesial surface of tooth No. 7. Furthermore, minimal soft tissue recession had occurred during the year following surgery.
In the esthetic zone, great lengths are taken to avoid procedures that may result in gingival recession. Patients must be informed (and provide informed consent) that surgical interventions may result in esthetic issues to varying degrees. The treatment of peri-implantitis requires thorough debridement and implant surface cleansing, which is virtually impossible without flap reflection. Surgical acumen, taking great care to avoid vascular trauma, is a necessity. Incision design, gentle soft-tissue handling, and tension-free closure can minimize postoperative recession. Support of the overlying soft tissues from the graft is also critical. The placement of the bone graft particulate should not only fill the defect but also be of sufficient thickness to support the flap and prevent collapse into the defect. To avoid soft tissue recession and esthetic failure, most researchers advocate for a minimal buccal bone thickness of 1.5 mm to 2.0 mm.
It is also important to consider the thickness of the mucosa. Linkevicious and colleagues demonstrated that a thicker peri-implant mucosa results in less crestal bone remodeling. Patients with thin, scalloped, periodontal phenotypes are more susceptible to recession. Utilizing a dermal allograft not only as a membrane for guided tissue/bone regeneration but also as a soft tissue thickening device may be advantageous in terms of preserving soft tissue and preventing recession.
Conclusion
This case report demonstrates the successful management of peri-implant disease in the esthetic zone. The mucosal recession associated with any surgical treatment was of primary concern. The presence of keratinized mucosa was viewed as a positive finding due to the concern that implants without a minimal zone of keratinized mucosa may be more susceptible to inflammation.9,10 Sculean and colleagues concluded that, in zones of minimal keratinized mucosa, subepithelial, connective grafts combined with coronally-advanced flaps as well as guided bone regeneration are capable of improving lesions of peri-implant recession.11 Although the underlying bone loss was substantial, the implant treated in this case report did not present with significant mucosal recession. This was seen as advantageous because both graft/membrane coverage and initial flap repositioning could be accomplished without periosteal releasing to coronally-advance the flap. Preservation of esthetics had to be respected to satisfy the patient’s expectations for treatment. Because the affected implant was not mobile, and the patient was not aware of the presence of disease until it was detected on clinical probing and radiographic exam, any compromise in esthetics could lead to patient management issues postoperatively. By augmenting the osseous defect with a biologically-enhanced bone graft, support for the overlying soft tissues was provided. The adjunctive use of a dermal allograft served two purposes. By utilizing allogeneic dermis as a barrier membrane, the peri-implant soft tissue is made thicker, possibly improving the resistance to mucosal recession. Also, because dermal allografts consist of natural collagen, cell-occlusive membrane function is provided for the underlying bone graft material.12
Although this single case report had a promising outcome, further clinical research is required before deriving conclusions regarding the most efficacious manner of treating these types of peri-implant lesions, especially in the esthetic zone.
References
1. Albrektsson T Isidor F. Consensus report of session IV. In: Lang NP Karring T (eds), Proceedings of the First European Workshop on Periodontology. London: Quintessence, 1994:365-369.
2. Berglundh T, Persson L, Klinge B. A systematinc review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontal. 2002;29(Suppl 3):197-212.
3. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533-540.
4. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: Clinical results. J Clin Periodontol. 2009;36(7):604-609.
5. Schwarz F, Bieling K, Bonspann M, Latz T, Becker J. Nonsurgical treatment of moderate and advanced periimplantitis lesions: a controlled clinical study. Clin Oral Investig. 2006;10(4):279-288.
6. Khoury F, Buchmann R. Surgical therapy of peri-implant disease: A 3-year follow-up study of cases treated with 3 different techniques of bone regeneration. J Periodontol. 2001;72(11):1498-1508.
7. Wiltfang J, Zernial O, Behrens E, Schlegel A, Warnke PH, Becker ST. Regeneration treatment of peri-implantitis bone defects with a combination of autologous bone and demineralized xenogeneic bone graft: A series of 36 defects. Clin Implant Dent Relat Res. 2012;14(3):421-427.
8. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitits with a regenerative approach: a consecutive series of 51 treated implants with a 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012; 32(1):11-20.
9. Warrer K, Buser D, Lang NP, Karring T. Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa. An experimental study in monkeys. Clin Oral Implants Res. 1995;6(3):131-138.
10. Wennström JL, Derks J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin Oral Implants Res. 2012;23(suppl 6):136-146.
11. Sculean A, Chappuis V, Cosgarea R. Coverage of mucosal recessions at dental implants. Periodontol 2000. 2017; 73(1):134-140.
12. Levin BP. The dual function of a dermal allograft in immediate implant therapy. Int J Periodontics Restorative Dent. 2015;35(4):507-513.
About the Author
Barry P. Levin, DMD
Clinical Associate Professor
University of Pennsylvania
Philadelphia, Pennsylvania
Private Practice
Jenkintown, Pennsylvania