Treatment of a Large Osseous Defect at Time of Extraction and Implant Placement
NovaBone offers excellent handling and placement with complete replacement
Osseous defects resulting from periodontitis or from prior extracted teeth can complicate implant placement and/or restorative treatment. Grafting of these sites can develop better ridge morphology for implant placement or to support either fixed or removable prosthetics.
Suitable graft materials range from the patient’s own bone from a different site to packaged human and bovine materials to synthetic graft products. Use of host bone is often limited to smaller defects due to volume considerations. When the defect is large, graft products are often the appropriate choice.
Packed graft products have been available as particles that are rehydrated either with sterile saline or the patient’s blood products and then placed into the site. The drawback to these materials is often they are difficult to contain and shape, especially in defects where only an osseous wall is present at the defect’s base. In recent years, putty formulations of graft materials have been introduced that make handling and placement easier. The authors have found that these materials stiffen at body temperature, allowing them to be shaped to the desired contours.
The authors will discuss a case using a bioglass putty (NovaBone®, NovaBone Products, www.novabone.com) combined with bioglass morsels to fill a large defect in the mandible that had lost the buccal and lingual walls and shown a significant loss of crestal bone height.
NovaBone Grafting Material
NovaBone is a calcium phosphosilicate material with a proven ability to signal genetic pathways to accelerate natural bone growth.1 The bioengineered release of silicon (Si), calcium (Ca), and phosphate (P) ions results in the stimulation of genes that are known to be critical in the repair and regeneration of bone tissue.2 This process is osteoinductive.3 The controlled release of ions over time enhances cell signaling, with osteostimulation causing a catalytic response that accelerates the natural healing process, resulting in bone regeneration.4,5
This process enhances osseous grafting as follows. Ca and P ions are released along with soluble silica, forming a silica gel and hydroxycarbonate apatite layer. This creates an ideal environment for cellular attachment and for protein/growth factor absorption. Signaling and recruitment of osteoprogenitor cells results from formation of the reaction layers and the absorption of these organic molecules at the site.6 These cells form an early attachment and proliferation at the graft surface. Continuous release of Ca and Si ions modulates the differentiation of the cells. This results in a population of osteoblasts and precursor cells that have the potential to become bone-forming cells. This accelerated early healing response results in bone formation on NovaBone particle surfaces at rates equivalent to autografts. Studies have shown particle absorption keeping pace with bone remodeling; the cumulative effect is an increased number of cells capable of dividing and forming new bone and healing tissue.7-9 Particles of NovaBone completely resorb over approximately a 6-month period and are replaced by native bone. Histology studies are unable to identify any particles at periods of 9 months to 1 year.10-12
NovaBone oral products have been researched for more than 20 years.13-15 The bioglass graft material is available as a putty and as morsels that can be used alone or mixed together. Incorporation of the morsels into the putty creates a stiffer material, allowing the practitioner to mold it to achieve three-dimensional (3D) shapes to tent the soft tissue when needed.
Case Presentation
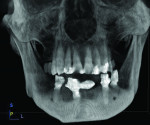
A 64-year-old man presented desiring treatment for failing mandibular dentition. Periapical radiographs were taken to evaluate the periodontal and structural condition of the mandible (Figure 1). Teeth Nos. 20 through 22 and Nos. 26 through 28 remained. A fixed porcelain-fused-to-metal bridge was present using Nos. 22, 26, and 27 as abutments.
Treatment Plan
Poor marginal adaption was noted on the bridge abutments. Mobility was moderate to severe in the remaining mandibular dentition, except for teeth Nos. 20 and 21. Radiographically, bone loss was noted as follows: 60% on No. 22 with an angular defect towards the midline, 90% on No. 26, 60% on No. 27 with a mesial defect, and 60% on No. 28. The patient was informed of the poor condition of the majority of the mandibular teeth and treatment options were discussed. These included either a conventional full denture or placement of implants and restoration with a fixed prosthesis.
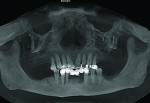
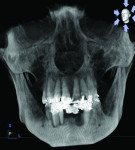
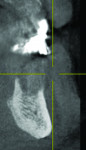
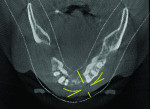
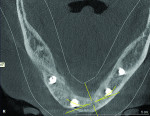
The patient expressed a desire for a fixed approach to treat the mandible. A cone-beam computed tomography (CBCT) scan was taken (Galileos, Sirona, www.sirona.com) to evaluate the existing bone in 3D to plan where implants could be placed and determine what additional procedures would be required (Figure 2). The CBCT scan confirmed the large osseous defect visualized in the periapical radiographs (Figure 3). Further analysis of sagittal view revealed a defect that had lost both the buccal and lingual walls, as well as a decrease in height almost to the apex of adjacent tooth No. 22 (Figure 4). An axial view further confirmed the extent of the defect, which was most likely associated with periodontal disease and the incomplete healing of site Nos. 23 and 24 after extraction (Figure 5).
After extraction of all remaining mandibular teeth and curettage of the extraction sockets and osseous defects, implants would be placed immediately with a spread between teeth Nos. 19 and 28 based on available bone in both height and width of ridge. The osseous defects remaining would be filled with graft material and the soft tissue closed. An immediate screw-retained provisional prosthesis would be placed if adequate insertion torque was present on all implants. The implants would be allowed to fully integrate and osseous grafts would organize into bone.
Clinical Protocol
Impressions were taken of the existing arches and occlusal records captured. These were sent to the lab for fabrication of a full denture. To act as a guide for implant placement, a 3/32” twist drill was used to place guide holes through the provisional prosthesis in the sites determined by the CBCT scan.
At the surgical appointment, local anesthetic was administered using an infiltration technique. The remaining mandibular teeth were atraumatically extracted. A crestal incision was made distal to the intended site of the posterior left implant and extended to distal of the intended right posterior implant. A full thickness flap was elevated. The extraction sockets and osseous defect in the anterior was curetted, removing all granulation tissue down to what appeared to be healthy bone.
The provisional prosthesis was placed and a 2.0-mm pilot drill was introduced through each of the four guide holes into the underlying bone to verify position. The prosthesis was removed and sequential drills were used to prepare the sites for the intended implants. Implants selected were Tapered ExHex implants (4.0-mm diameter) in the anterior left site and Co-Axis implants (5.0-mm diameter) (Keystone Dental, www.keystonedental.com) in the bilateral posterior and anterior right sites. The Co-Axis implants (12°) were selected for these sites because the anatomy present would allow a longer implant length while correcting the angulation needed for ease of prosthetic restoration. The tapered implant was selected based on the available anatomy present at this site.
Following site preparation, implants and healing abutments were placed. NovaBone putty and NovaBone morsels were mixed in a 50/50 ratio to provide more substance and a stiffer product to tent the soft tissue over the large anterior defect. The graft mixture was placed to completely fill the anterior large defect. A piece of resorbable collagen membrane was placed over the graft in the anterior and the full-thickness flap was closed over the site using non-resorbable PTFE monofilament suture to achieve primary closure.
The healing abutments were removed and a titanium temporary cylinder was affixed to each implant. Polymethyl methacrylate was mixed to a viscous consistency and injected into the gap between the temporary cylinder and provisional prosthesis. Upon setting, additional acrylic was injected into the tissue side of the prosthesis to fully lock the temporary cylinders to the prosthesis. Following setting, the flanges on the provisional prosthesis were removed with an acrylic bur to convert the prosthesis into a screw-retained fixed bridge. A carbide bur was then used to shorten the temporary cylinders that projected superior to the provisional’s occlusal surface. The prosthesis was attached to the implants using fixation screws tightened to 30 Ncm. A piece of PTFE tape was formed into a ball and placed into the superior aspect of the temporary cylinder, followed by flowable composite (Flow-It®, Pentron Clinical, www.pentron.com), and then light-cured. Occlusion was checked and adjusted as appropriate.
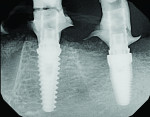
The patient was seen 3 weeks following treatment to check healing of the soft tissue and for suture removal. Periapical radiographs were taken that showed some evidence of the graft material in the sites and seating of the provisional prosthesis fully on the implants with no intervening gaps (Figure 6 and Figure 7).
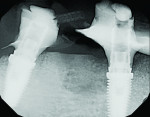
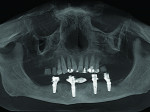
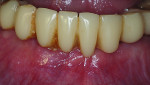
Six months following treatment, integration of the implants and fill of the osseous defect was evaluated in preparation for the final restorative phase. A CBCT was taken and a reconstructed panoramic view showed an absence of the previous defect (Figure 8). A periapical radiograph demonstrated complete osseous fill and blending of the graft with the adjacent native bone (Figure 9). Additional views demonstrated the implants surrounded by bone and elimination of the grafted defects with a good blend with the adjacent bone (Figure 10 and Figure 11). The cross-sectional (sagittal) view demonstrated fill of the anterior osseous defect with replacement of bone to the normal height and width of the ridge (Figure 12); this was compared to the pretreatment image (Figure 4). The mandible at the left of the midline demonstrated normal contours and healthy non-inflamed soft tissue overlay the grafted area (Figure 13).
Discussion
The selection of the proper osseous grafting material is case dependent. Osseous graft materials had been previously available in a dry granular form, which complicated placement in large defects with few walls to contain the graft. Grafts placed with these materials tended to migrate and disperse, and building either height or width could be challenging. In recent years, companies have introduced graft materials in “putty” form, making placement easier and letting the practitioner shape the graft to ideally fill the defect and build up the area.
Demineralized human bone (allograft) had been the gold standard in the past. Bovine products (xenografts) had gained popularity as well, but studies have found residual particles at one 1 year or more from graft placement, and they may never fully convert to native bone.16,17 Early versions of synthetic materials also suffered from failure to fully resorb over time. But changes in chemistry have allowed development of bioglasses that are fully resorbable over a period of 6 to 9 months and are replaced by native bone.18
Graft materials can act like a scaffold and allow the body to slowly replace them with bone (osteoconduction) or they can stimulate bone formation (osteoinduction). Bioglasses, such as the NovaBone products used in this case, have osteostimulatory properties and can induce bone formation as well as speed defect fill and organization.19-21
Conclusion
Decisions on whether to graft defects and extraction sockets can be a clinical challenge. Small defects typically do not need grafting, as the clot that forms in these areas following curettage can adequately achieve bone fill. However, large defects typically do not completely fill without grafting. If grafting is not performed, they may demonstrate continuing defects over time, complicating and compromising restorative treatment. In addition, extraction sockets are aided by filling them with a graft material, especially if an implant is to be placed in or adjacent to the site.
NovaBone has, through 20 years of research and development into bioglasses, developed a fully resorbable grafting material that has osteoinductive and osteostimulatory properties available in an easy-to-handle putty formulation. In this case, these properties allowed 3D rebuilding of the lost osseous ridge back to dimensions present prior to formation of the defect.
References
1. Carinci F, Palmieri A, Martinelli M, et al. Genetic portrait of osteoblast-like cells cultured on Bioactive Glass. J Oral Implantol. 2007;33(6):327-333.
2. Zhai W, Lu H, Wu C, et al. Stimulatory effects of the ionic products from Ca-Mg-Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomater. 2013;9(8):8004-8014.
3. Galindo-Moreno P, Avila G, Fernández-Barbero JE, Mesa F, et al. Clinical and histologic comparison of two different composite grafts for sinus augmentation: a pilot clinical trial. Clin Oral Implants Res. 2008;19(8):755-759.
4. Li H, Xue K, Kong N, et al. Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone marrow stromal cells. Biomaterials. 2014;35(12):3803-3818.
5. Xynos ID, Edgar AJ, Buttery LD, et al. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun. 2000;276(2):461-465.
6. Bombonato-Prado KF, Bellesini LS, Junta CM, et al. Microarray-based gene expression analysis of human osteoblasts in response to different biomaterials. J Biomed Mater Res A. 2009;88(2):401-408.
7. Taylor JC, Cuff SE, Leger JP, et al. In vitro osteoclast resorption of bone substitute biomaterials used for implant site augmentation: a pilot study. Int J Oral Maxillofac Implants. 2002;17(3):321-330.
8. Gatti AM, Simonetti LA, Monari E, et al. Bone augmentation with bioactive glass in three cases of dental implant placement. J Biomater Appl. 2006;20(4):325-339.
9. Murphy S, Wren AW, Towler MR, Boyd D. The effect of ionic dissolution products of Ca-Sr-Na-Zn-Si bioactive glass on in vitro cytocompatibility. J Mater Sci Mater Med. 2010;21(10):2827-2834.
10. Loty C, Sautier JM, Tan MT, et al. Bioactive glass stimulates in vitro osteoblast differentiation and creates a favorable template for bone tissue formation. J Bone Miner Res. 2001;16(2):231-239.
11. Norton MR, Wilson J. Dental implants placed in extraction sites implanted with bioactive glass: human histology and clinical outcome. Int J Oral Maxillofac Implants. 2002;17(2):249-257.
12. Galindo-Moreno P, Avila G, Fernández-Barbero JE, et al. Clinical and histologic comparison of two different composite grafts for sinus augmentation: a pilot clinical trial. Clin Oral Implants Res. 2008;19(8):755-759.
13. Vrouwenvelder WC, Groot CG, de Groot K. Histological and biochemical evaluation of osteoblasts cultured on bioactive glass, hydroxyapatite, titanium alloy, and stainless steel. J Biomed Mater Res. 1993;27(4):465-475.
14. Price N, Bendall SP, Frondoza C, et al. Human osteoblast-like cells (MG63) proliferate on a bioactive glass surface. J Biomed Mater Res. 1997;37(3):394-400.
15. Xynos ID, Hukkanen MV, Batten JJ, et al. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67(4):321-329.
16. Galindo-Moreno P, Avila G, Fernández-Barbero JE, et al. Evaluation of sinus floor elevation using a composite bone graft mixture. Clin Oral Implants Res. 2007;18(3):376-382.
17. Galindo-Moreno P, Avila G, Fernández-Barbero JE, et al. Clinical and histologic comparison of two different composite grafts for sinus augmentation: a pilot clinical trial. Clin Oral Implants Res. 2008;19(8):755-759.
18. Hamadouche M, Meunier A, Greenspan DC, et al. Long-term in vivo bioactivity and degradability of bulk sol-gel bioactive glasses. J Biomed Mater Res. 2001;54(4):560-566.
19. Bosetti M, Cannas M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials. 2005;26(18):3873-3879.
20. Phan PV, Grzanna M, Chu J, et al. The effect of silica-containing calcium-phosphate particles on human osteoblasts in vitro. J Biomed Mater Res. 2003;67(3):1001-1008.
21. Xynos ID, Hukkanen MV, Batten JJ, et al. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67(4):321-329.
For more information, contact:
NovaBone Products
904-807-0140
www.novabone.com