Treatment Options for Hypoplastic and Hypocalcified Permanent Molars in Children
Painful tooth sensitivity caused by these enamel defects is a main target for treatment
Molars that are malformed due to enamel hypoplasia, enamel hypocalcification, or amelogenesis imperfecta routinely challenge dentists who treat children.1-10 These teeth often wear or fracture and are prone to caries, and many malformed posterior teeth are concurrently hypoplastic and hypocalcified. Patients with these conditions often complain about tooth sensitivity because abnormal or missing enamel can mean vital dentin may not be insulated from the stimuli associated with cold or hot food or liquids, sweet foods, or air. The resulting tooth sensitivity can be a continuing source of discomfort for the patient.
Definitions and Diagnosis
The nomenclature regarding malformed teeth can be confusing. Enamel hypoplasia is generally defined as a defect in the enamel characterized by a lack of tooth contact, a rapid breakdown of the occlusal surfaces, and a yellowish-brown stain where the dentin is exposed. In hypoplasia the enamel is hard, but it is also thin and deficient in quantity. The condition is a result of defective enamel matrix formation.11
Enamel hypocalcification is characterized by soft and undercalcified enamel that is opaque in appearance but normal in quantity. In hypocalcification the surfaces also wear down quickly and are more susceptible to caries; the teeth are also chalky in consistency. Enamel hypocalcification is caused by defective maturation of ameloblasts.11
Both enamel hypoplasia and enamel hypocalcification may be seen in teeth with amelogenesis imperfecta. Amelogenesis imperfecta is inherited as an autosomal-dominant trait and classified according to its severity: enamel hypoplasia, enamel hypocalcification, or agenesis, in which there is a complete lack of enamel.11
Amelogenesis imperfecta is genetically based and has many manifestations.10 While in some cases the etiology of enamel hypoplasia and enamel hypocalcification may be genetic, usually the dentist can only guess the cause of the defect. Some dentists tell patients or parents that the tooth abnormality resulted from a fever, the use of antibiotics in early childhood, or from childhood diseases. Such conjectures usually do not appear to be true because the child will have one permanent first molar, or even several, with severe enamel hypoplasia and enamel hypocalcification, whereas the other permanent first molars and all of the other permanent teeth that had undergone amelogenesis at the same developmental time are completely normal. It is therefore correct to refer to most enamel hypoplasia and enamel hypocalcification defects of permanent molars as “idiopathic” if no genetic etiology can be verified.
Treatment of Enamel Malformations
Some small hypoplastic and hypocalcified defects do not require any treatment. If an enamel malformation is located in an area of low stress, there is no caries present, and the patient reports no tooth sensitivity, a reasonable treatment approach is to continually re-evaluate the status of that tooth, periodically apply fluoride, and teach the parent and child how to keep the tooth clean.
When the tooth structure breaks down from usual wear and tear, a caries lesion is detected, or if the patient complains of sensitivity, some type of clinical intervention is required. The methods for treating malformed permanent molars can summarized as follows:
• Application of a resin-bonded sealant to eliminate sensitivity
• Restoration using a resin-based composite alone
• Restoration using a glass-ionomer system, such as a powder/liquid or an encapsulated, chemically hardened glass-ionomer cement
• Restoration using a stratified resin-based composite overlying RMGI cement13-16
• Complete interim coronal coverage using preformed stainless-steel crown forms.17,18
The treatment goals for hypoplastic/hypocalcified permanent molars in young patients include restoring the tooth so that it no longer has sensitive dentin; re-creating coronal form to re-establish normal function, occlusion, and proximal contacts; eliminating caries infection and preventing further caries involvement; and preserving the tooth structure so that future preparation for precision indirect restoration will not be compromised.
There are cases in which a permanent first molar is so severely malformed, either with or without caries involvement, that the best treatment is extraction with the goal of the adjacent permanent second molar migrating mesially to replace the missing tooth.19,20 However, case selection is critically important in these circumstances. Ideally, the second molar would have minimal root development and appear on the radiograph to have a slight mesioangular position. In addition, the third molar also would have to be evident on the radiograph, so that it too has a high likelihood of replacing the second molar by natural shifting. Severely malformed second molars can be removed if there is adequate assurance that the third molar is evident and can migrate mesially into position. Consultation with an orthodontic specialist is helpful in these cases. When treatment planning, the clinician must weigh the risks of the replacement teeth also being malformed, and parents need to know that orthodontic intervention may be needed in the future.
Case Examples
Treatment modes for the repair of hypoplastic and hypocalcified permanent molars are detailed below. All of the patients described were between 7 and 11 years old at the time of treatment.
Case 1
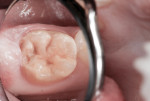
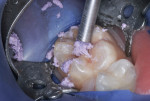
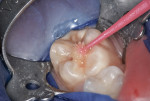
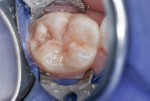
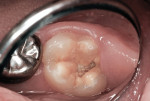
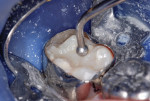
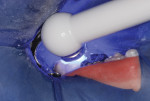
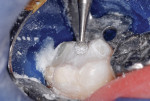
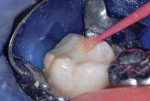
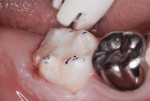
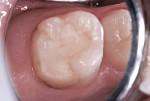
This patient reported intense sensitivity to cold liquids and air inspiration of the left maxillary and mandibular permanent first molars. The right permanent first molars had no enamel abnormalities. The left molars had no evident caries infection and no breakdown of enamel (Figure 1). After local anesthetic injections and rubber dam placement, the occlusal surface was cleaned with a prophy paste and a small brush (Figure 2). (This author has found no difference if a fluoride paste is used in this step, as long as acid-etching is sufficient.) A self-etching bonding agent (Adper™ Prompt™ L-Pop™ Self-Etch Adhesive, 3M ESPE, www.3mespe.com) was applied and gently rubbed on the tooth surface for 20 seconds. A coating of clear resin sealant was then applied and photopolymerized (Figure 3). The maxillary left first molar was treated in the same manner. The patient and parents have reported no sensitivity associated with either tooth 6 months after treatment (Figure 4).
Case 2
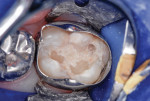
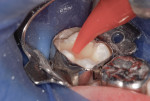
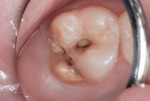
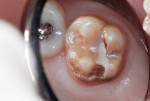
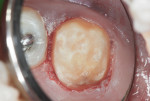
A 7-year-old had a chief complaint of “my new tooth hurts.” The mother related that her son recently complained of sensitivity whenever he consumed cold fluids. Inspired air also elicited discomfort. A large portion of the occlusal surface of the partially erupted mandibular right permanent first molar was hypoplastic and hypocalcified. In addition, a caries lesion had developed in the malformed enamel (Figure 5). After an inferior alveolar block anesthetic injection, an oversized orthodontic band was placed to stabilize the rubber dam retainer and retract the operculum covering the partially erupted tooth. The carious tooth structure and most of the malformed enamel was cut away with a high-speed, water-cooled cylindrical diamond bur (Figure 6).
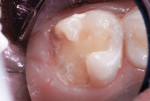
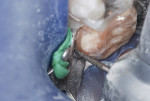
A RMGI liner/base material (Vitrebond™ Plus, 3M ESPE) was injected and spread throughout the preparation (Figure 7). Care was taken to eliminate any air bubbles that would create voids once the material hardened. Using a large, inverted cone bur at slow speed, the RMGI liner was cut so that it covered the natural dentin layer but did not overlap the enamel (Figure 8).
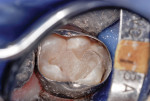
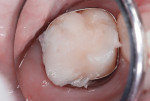
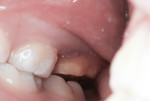
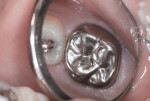
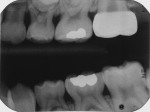
The first portion of nano-ionomer restorative cement (Ketac™ Nano, 3M ESPE) was then injected, compressed into the preparation, and light-cured at 1,100 mW/cm2 for 20 seconds (Figure 9). Another portion of cement was then injected to overfill the cavity preparation, and the curing light was applied again for 20 seconds (Figure 10). After the orthodontic band was removed, the cement was cut to contour with slow-speed round diamond burs of several sizes (Figure 11). The trimmed occlusal surface was then treated with a self-etching resin-bonding agent (Adper Prompt L-Pop) for margin and surface sealing (Figure 12). A final light exposure of 30 seconds was used to ensure through-and-through cement hardening. After the rubber dam was removed, occlusal contacts were evaluated with articulating paper (Figure 13). The tooth was photographed 34 months after treatment (Figure 14).
Case 3
This maxillary molar was restored in the same manner used in Case 2. Hypoplastic and hypocalcified defects complicated by caries affected the occlusal, distal, and lingual surfaces (Figure 15). The tooth will require a precision-type onlay or three-quarter crown in the patient’s late teenage or adult years. With local anesthesia, the malformed and carious tooth structure was cut away, and a RMGI liner placed (Figure 16). The tooth was restored with the nano-ionomer cement and light-cured in three increments. An orthodontic band served as a matrix (Figure 17). The nano-isomer (Ketac™ Nano, 3M ESPE) was intact 36 months postoperatively (Figure 18).
Case 4
This first molar had severe enamel hypoplasia and enamel hypocalcification malformation. It had been treated elsewhere using resin-based composite and residual restorative material was evident. The child reported intense tooth sensitivity with cold and hot stimulus, air inspiration, and when he ate sweets. Because of the pervasive enamel malformation, significant sensitivity, and caries, full-coverage repair using a preformed stainless-steel crown was required (Figure 19).17,18 Using local anesthesia, the crown was reduced in miniature, conservatively, so that future preparation for a precision long-term crown repair would not be compromised (Figure 20 through Figure 23). The crown is shown clinically and on a bitewing radiograph, 18 months after placement (Figure 24).17,18
Conclusion
After several decades of using adhesively bonded dental restorative materials for the restoration of permanent posterior teeth with hypoplastic or hypocalcified malformation of the enamel, the author has made certain observations to share with fellow clinicians.
First, routine bonding of resin-based composite to hypoplastic or hypocalcified enamel using the acid-etch method is not predictable or reliable over the long term. That is especially true in areas of high stress. Debonding is often seen and the surrounding tooth structure may crumble because of its weakened state. The breakdown of peripheral enamel as a result of caries is common (Case 4). However, resin-based composite does hold up well if the material at the margins is bonded to sound, unaffected enamel peripheral to the defective enamel. Being as conservative as possible, internal mechanical retention augments retention achieved with the standard acid-etch bonding protocol.
The second observation is that the glass-ionomer systems—with their chemical bonding ability, fluoride content, coefficients of thermal expansion similar to that of tooth structure, and biocompatibility—can hold up well as long as they are not placed in regions of high occlusal load or masticatory stress. However, they do not have the physical strengths and wear resistance of resin-based composites. A modified mechanical retention-form preparation with undercuts is useful, as it is with resin-based composites, but the conservation of tooth structure must also be considered.
Although the nano-ionomer appears to be the RMGI that has physical properties closest to those of the resin-based composites, it does not have the physical strengths, such as resistance to wear, of the resin-based composites. But, keep in mind that RMGI cements can be overlaid with bonded resin-based composite material after serving many years as an ideal “interim” restorative material.21 Whenever possible, stratifying RMGIs and resin-based composites is perhaps the best means of optimizing the best qualities of both restoratives.13-16
Another observation is that manufacturers have improved the properties and handling characteristics of some non-light–cured glass-ionomer cements (not pictured in this article) and they can be used for interim restoration of certain malformed molars. Although they do not have the advantage of rapid “on command” hardening or some of the physical characteristics of their resin-modified counterparts, they are much more durable and faster-setting than the original glass ionomers.12
Some may question why a RMGI liner/base material was used underneath the nano-ionomer material in Case 2 and Case 3. The Vitrebond Plus material has excellent wettability, it spreads into all undercuts easily, and since 1987, it has an excellent clinical history. Using the liner ensures that the entire preparation is sealed and, if future marginal leakage occurs, another layer of bonded material serves as an “internal guardian” of any pathway to the dentin and pulp. The material is known to have an antibacterial effect because of its fluoride content, and the dentin bond of RMGIs do not hydrolyze as resin/dentin bonds do.15 In addition, the nano-ionomer does not have a chemical hardening component like some RMGI cements. By placing an initial deep liner/base, penetration with the curing light is more assured.
The decision to restore a malformed permanent molar using a preformed stainless-steel crown can be a difficult one to make. The molar in Case 3 could have been repaired using a stainless-steel crown. However, because the two buccal cusps and the load-bearing mesiolingual cusp were sound, the nano-ionomer material was selected for the sake of conserving tooth structure. No additional treatment has been needed in 3 years. In general, if 50% or more of an anatomical crown is sound, bonded adhesive restoration augmented by judicious mechanical retention form should be the first choice when repairing malformed permanent molars. The procedure for restoring permanent molars with preformed stainless-steel crowns is significantly different from the procedure for restoring a primary molar. Conservation of tooth structure is paramount, marginal adaptation of the crown form requires careful crimping, finishing, and polishing, and a precementation bitewing radiograph is recommended to ensure proximal fit.17,18
References
1. Pindborg JJ. Aetiology of developmental enamel defects not related to fluorosis. Int Dent J. 1982;32(2):123-134.
2. Suckling GW. Developmental defects of enamel—historical and present-day perspectives of their pathogenesis. Adv Dent Res. 1989;3(2):87-94.
3. Hargreaves JA, Cleaton-Jones PE, Williams SD. Hypocalcification and hypoplasia in permanent teeth of children from different ethnic groups in South Africa assessed with a new index. Adv Dent Res. 1989;3(2):126-131.
4. van Amerongen WE, Kreulen CM. Cheese molars: a pilot study of the etiology of hypocalcifications in first permanent molars. ASDC J Dent Child. 1995;62(4):266-269.
5. Jälevik B, Norén JG. Enamel hypomineralization of permanent first molars: a morphological study and survey of possible aetiological factors. Int J Paediatr Dent. 2000;10(4):278-289.
6. Croll TP. Restorative options for malformed permanent molars in children. Compend Contin Educ Dent. 2000;21(8):676-678, 680, 682.
7. Leppäniemi A, Lukinmaa PL, Alaluusua S. Nonfluoride hypomineralizations in the permanent first molars and their impact on the treatment need. Caries Res. 2001;35(1):36-40.
8. Weerheijm KL, Groen HJ, Beentjes VE, Poorterman JH. Prevalence of cheese molars in eleven-year-old Dutch children. ASDC J Dent Child. 2001;68(4):259-262.
9. Weerheijm KL, Jälevik B, Alaluusua S. Molar-incisor hypomineralisation. Caries Res. 2001;35(5):390-391.
10. Wright JT. Developmental Defects of the Teeth. University of North Carolina at Chapel Hill website. www.dentistry.unc.edu/research/defects/pages/ai.htm. Accessed May 29, 2014.
11. Mosby’s Medical Dictionary. 8th ed. St. Louis, MO: Elsevier; 2009.
12. Croll TP. Rapid-setting encapsulated glass-ionomer restorative cement. Compend Contin Educ Dent. 2001;22(5):442-446, 448.
13. Croll TP. Replacement of defective Class I amalgam restorations with stratified glass ionomer-composite resin materials. Quintessence Int. 1989;20(10):711-716.
14. Croll TP. Class I composite resin restoration. J Esthet Dent. 1992;4(5):148-153.
15. Ruiz JL, Mitra S. Using cavity liners with direct posterior composite restorations. Compend Contin Educ Dent. 2006;27(6):347-351; quiz 352.
16. Croll TP, Cavanaugh RR. Posterior resin-based composite restorations: a second opinion. J Esthet Restor Dent. 2002;14(5):303-312.
17. Croll TP, Castaldi CR. The preformed stainless steel crown for restoration of permanent posterior teeth in special cases. J Am Dent Assoc. 1978;97(10):644-649.
18. Croll TP. Preformed posterior stainless steel crowns: an update. Compend Contin Educ Dent. 1999;20(2):89-92, 94-96, 98-100 passim; quiz 106.
19. Penchas J, Peretz B, Becker A. The dilemma of treating severely decayed first permanent molars in children: to restore or to extract. ASDC J Dent Child. 1994;61(3):199-205.
20. Sandler PJ, Atkinson R, Murray AM. For four sixes. Am J Orthod Dentofacial Orthop. 2000;117(4):418-434.
21. Croll TP, Cavanaugh RR. Resurfacing resin-modified glass-ionomer restorations. Inside Dentistry. 2009;5(1):82-83.
About the Author
Theodore P. Croll, DDS
Private Practice
Doylestown, Pennsylvania
Affiliate Professor
Department of Pediatric Dentistry University of Washington School of Dentistry
Seattle, Washington
Adjunct Professor
Department of Pediatric Dentistry, University of Texas Health Science Center at San Antonio
San Antonio, Texas