Grafting of an Extracted Maxillary First-Molar Socket
Using a new, flexible resorbable collagen membrane for ridge preservation in advance of implant placement.
By Robert A. del Castillo, DMD, PA
In the first year after extraction, major changes take place at the extraction site.1 Alveolar width reductions of between 5 mm and 7 mm have been reported, with approximately two thirds of that reduction occurring within the first 3 months.2 To facilitate later placement of an endosseous implant, ridge-preservation procedures using graft material in conjunction with a barrier membrane placed to prevent infiltration of gingival fibroblasts and/or epithelial cells have been developed, reportedly improving both ridge height and width.3
While membrane exposure has been one of the most frequent complications of guided bone regeneration (GBR) procedures, a clear consensus does not exist regarding the significance of such exposure for regenerative outcomes. Several clinical trials have shown worse responses when submerged membranes have become exposed,4,5 and one meta-analysis found that membrane exposure during healing had a major negative effect on GBR around dental implants.6 Other researchers7,8 have found that membrane exposures lead to the need for more frequent follow-up visits. Patient management becomes more critical.
Moreover, when carrying out ridge preservation in anticipation of later implant placement, primary closure over the graft material may not be possible. In such cases, the use of a resorbable membrane with good tissue-response properties is important.
This case report presents treatment of a patient whose maxillary first molar was extracted in preparation for treatment with two-stage implant placement. To preserve the ridge, the socket was filled with a xenograft and covered with an OsseoGuard Flex™ Membrane (BIOMET 3i, www.biomet3i.com) that was intentionally left partially exposed.
Flexible Collagen
The OsseoGuard® Membrane is fabricated from bovine Type I Achilles tendon collagen sourced from closed herds for increased security. Membranes made of bovine collagen do not elicit an antibody response9 when used for GTR.
Although resorbable collagen placed within periodontal tissue is normally degraded by endogenous collagenase into carbon dioxide and water,10 cross-linkage of the collagen fibers can reduce the rate of degradation.11 Cross-linking is a laboratory modification of the collagenous matrix that stabilizes the collagen fibers; it maintains membrane integrity after placement,12 lengthening the resorption rate. The cross-linked structure of all OsseoGuard Membranes results in a long resorption profile that is ideal for GBR procedures. The manufacturing process also allows for the formation of a dense fibrillar matrix that provides strength for tacking or suturing, if desired.
The new OsseoGuard Flex Membrane is made from bovine dermis, which includes both Type I and Type III collagen. While the collagen fibers in this membrane are cross-linked, the linkage is slightly different than that of the original version, changing the membrane’s handling characteristics and giving it a spongier tactile quality. Because it is more flexible, it conforms readily to ridge contours.
Indications for collagen membranes include extraction sites, localized ridge augmentation, future implant site preparation, peri-implant bone defects around implants, bone regeneration after root resection, and sinus membrane perforations.
Case Report
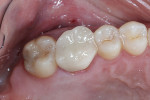
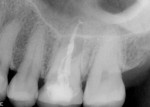
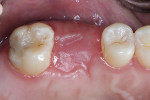
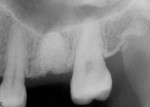
The patient was a 42-year-old woman who presented complaining that her left maxillary first molar had become extremely sensitive in the wake of recent endodontic treatment and delivery of a provisional crown. Her medical history was non-contributory, and oral and radiographic examination revealed the presence of a persistent periapical lesion and broken file (Figure 1 and Figure 2). Although the height of her alveolar ridge at the molar site was estimated to be 10 mm, the patient’s history of chronic endodontic problems was judged to be a contraindication for immediate implant placement after extraction of the failing tooth. Instead, the treatment plan called for placement of an implant only after the molar was extracted, the socket was grafted, and the grafted site was allowed to heal.
The patient provided informed consent, and on the day of surgery, the provisional crown was removed. In order to preserve the interdental papillae, a #557 straight-fissure crosscut carbide bur was used to section the tooth into three pieces that corresponded to each of the three roots. Each root section was then carefully extracted (Figure 3). The socket was meticulously cleaned using hand curettes, an ultrasonic scaler, and high-speed carbide burs. A small round bur was used to eliminate several bony projections from the ridge, and using a P24G periosteal elevator (Hu-Friedy, www.hu-friedy.com), a tunnel procedure was performed on the palatal and buccal aspects of the extraction socket to maintain vascularity and create a pouch into which the membrane could later be tucked.
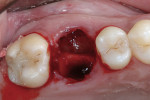
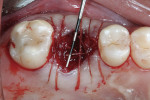
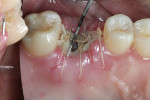
Xenograft particles were packed into the extraction site (Figure 4). An OsseoGuard Flex Membrane, which was 15 mm x 20 mm, was trimmed to fit over the graft site, leaving sufficient material to be tucked under the soft tissue. No tacking of the membrane was necessary. Instead, small releasing incisions were used to allow the soft tissue to be apically repositioned to maintain the esthetic position of the keratinized buccal tissue. Primary closure could not be achieved; coated VICRYL 4-0 undyed interrupted sutures (Ethicon Inc., www.ethicon360.com) secured the soft tissue (Figure 5).
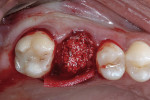
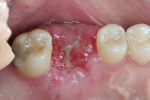
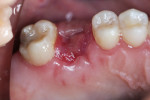
The patient was prescribed antibiotic, anti-inflammatory, and analgesic medications and instructed to avoid mastication, brushing, or flossing near the extraction site. She returned for follow-up examinations every week for the first month postoperatively. At each appointment, uneventful tissue healing was noted, with granulation tissue gradually filling in and covering the membrane (Figure 6, Figure 7, Figure 8 and Figure 9).
Four months after the extraction and grafting, a panoramic radiograph showed that although the granular nature of the graft material was still discernible, it was well contained, and the height was even with the crestal bone both mesial and distal to the extraction site (Figure 10). The patient was instructed to return 6 months postoperatively for implant placement within the regenerated ridge.
Conclusion
In the case presented here, grafting of the patient’s extracted maxillary first-molar socket with a xenograft and coverage of the graft site with an OsseoGuard Flex Membrane successfully occluded gingival and epithelial cells, enabling excellent preservation of the ridge. Although the membrane was left exposed, soft-tissue growth was swift and comprehensive, with no inflammation, laying the ground for future implant placement.
Disclosure
The author is a consultant for BIOMET 3i.
References
1. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17(1):21-27.
2. Schropp L, Wenzell A, Kostopoulos L, Karrin T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
3. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7):990-999.
4. Becker W, Dahlin C, Becker BE, et al. The use of e-PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: a prospective multicenter study. Int J Oral Maxillofac Implants. 1994;9(1):31-40.
5. Simion M, Baldoni M, Zaffe D. Guided tissue regeneration in osseointegrated implants. II: extraction sockets. Ital J Osseointegration. 1991;1:40-45.
6. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001;72(4):512-516.
7. Mellonig JT, Triplett RG. Guided tissue regeneration and endosseous dental implants. Int J Periodontics Restorative Dent. 1993;13(2):108-119.
8. Shanaman RH. A retrospective study of 237 sites treated consecutively with guided tissue regeneration. Int J Periodontics Restorative Dent. 1994;14(4):292-301.
9. Wang HL, Carroll MJ. Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int. 2001;32(7):504-515.
10. Hitti RA, Kerns DG. Guided bone regeneration in the oral cavity: a review. Open Pathol J. 2011;5:33-45.
11. Minabe M, Kodama T, Kougou T, et al. Different cross-linked types of collagen implanted in rat palatal gingiva. J Periodontol. 1989;60(1):35-43.
12. Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials. 2003;24(5):759-767.
About the Author
Robert A. del Castillo, DMD, PA
Private Practice
Miami Lakes, Florida