Oral Cancer Screening Aids
Michael A. Kahn, DDS
Early detection of oral cancer is the key to long-term survival, and comprehensive, effective screening is the basis of early detection. The definition of "at-risk" patients, who have a probability of developing oral cancer, has evolved to include more groups, and routine screening is being expanded for additional asymptomatic patients. In this environment, new and existing technologies are being implemented as adjunctive screening aids to assist the healthcare provider in identifying, evaluating, monitoring, and treating a growing number of patients.
These adjunctive screening aids are no substitute for the initial examination with direct incandescent light, dried-off cavity, and palpation with gloved hands. Nor are these methods an alternative to the "gold standard" biopsy and histologic analysis. Biopsy, whether laser, conventional, or punch, remains the most accurate diagnostic procedure.
However, healthcare practitioners face many real-life situations where an immediate biopsy is not practical or beneficial. In these cases, there are a number of appropriate intermediate procedures available to support the dentist’s observations and aid in monitoring the patient’s condition. This article will review some of these screening aids, their operation, efficacy, and function in a comprehensive diagnostic process.
ORAL CANCER RISK PROFILE
This year, there were approximately 30,990 new oral cancer diagnoses and 7,430 deaths.1 Oral cancer has three times the incidence and twice the death rate of cervical cancer.2 However, public awareness and education is lacking. While there is mainstream recognition and demand for screening breast and cervical cancers, most dental patients are not aware that they are being examined for oral cancer, nor do they understand the risk. In the case of some adjunctive screening methods, the procedure may not be reimbursed by certain medical insurance companies without an educational effort and higher patient demand.
Until a few years ago, patients considered at increased risk for developing oral cancer were those over the age of 40; however, the profile is expanding as more information becomes available.3 While preferably every dental patient should undergo routine visual screening, there are certain groups with additional risk factors. There is an increased risk for patients ages 18 to 39 who are sexually active. There is a higher risk for tobacco users over age 30. The highest risk is for patients over 40 with certain lifestyle factors, such as the use of tobacco and alcohol, as well as patients with a history of oral cancer.4 Even in the absence of any distinct oral condition, these identified groups should be carefully monitored with the most appropriate and efficacious technologies.
ROLE OF ADJUNCTIVE SCREENING AIDS
All screening begins with a patient history and a thorough visual examination with palpation, paying particular attention to highest-risk sites such as the lower lip, tongue, floor of the mouth, and the soft palate complex. Clinical judgment and experience is applied to differentiate leukoplakia, erythroplakia, and erythroleukoplakia; frictional abrasions and ulcers; and other oral conditions. The goal is to locate premalignant cells and remove lesions at mild to moderate dysplasia.
Once a lesion has been identified by visual examination, if the dentist is not 100% sure that it is benign, a biopsy is essential. After thoroughly documenting the findings and subsequent recommendation, additional follow-up with patients to verify that they took further action is advised.
If an immediate biopsy is not deemed crucial, the lesion should be re-evaluated in approximately 14 days for signs of change or improvement. In the meantime, the general dentist also has access to adjunctive screening technologies that may be used immediately to gather more information, especially if the patient is unlikely to return for the follow-up examination. These methods are also valuable in photographing and documenting the lesion at that moment for the patient’s records or for the oral surgeon.
To use these aids, a dentist does not need to anesthetize or cut patient tissue, which may be crucial for patients who cannot or will not undergo this procedure. Also, the dentist does not need to have oral pathology laboratory experience, as most dental schools have pathology services.
DEVELOPMENT
Often a procedure that is developed on the medical front is eventually applied to the oral cavity. In this case, the use of a Pap smear to screen for cervical cancer has been extended to the mouth. More than 55 million Pap smears are performed each year, resulting in an estimated 9,710 new cervical cancer diagnoses.2 This test is an acknowledged success, resulting in a 70% reduction in cervical cancer deaths in 10 years.2 Its use is now routine, requested by patients, and covered by insurance companies.
These procedures for the cervical mucosa have been successfully adapted for use in the oral cavity. Food and Drug Administration (FDA) approvals were often predicated on the approvals for cervical can-cer screening, and many of these devices now have indication statements claiming their use for the early detection of oral mucosal abnormalities.
EXFOLIATIVE CYTOLOGY
The earliest method of oral cancer screening, exfoliative cytology, was adapted from the Pap smear. Cells are collected by stroking the lesion with a tongue blade or cotton tip applicator, smearing them on a frosted glass slide, and fixing the sample with an alcohol-ether spray. The slide is shipped to an oral pathology laboratory, where it is stained, coverslipped, and analyzed by an oral pathologist.
As with similar collection methods, there are fundamental problems with the resulting sample that diminish its diagnostic value. An estimated up to 80% of the cells remain on the discarded collection device, and the cells that are obtained are only surface cells.5 Other elements, such as blood and debris, contaminate the sample. The manual method of smearing the slide results in layers of overlapping cells, reducing the clarity of the sample, and complicating the evaluation process. These factors limit the accuracy of exfoliative cytology, resulting in significant false-negatives and false-positives.6 However, at the time, it was the only adjunctive screening process available before proceeding to a biopsy.
BRUSH BIOPSY
The brush biopsy, introduced in the United States in 1999, was the next development in adjunctive screening. A patented collection device, in the form of a stiff, spiral-shaped nylon brush, is employed to improve the sampling process. The brush is pressed and twirled at the site of the suspicious lesion, and pinpoint bleeding spots indicate that it has penetrated the epithelial layers to the basement membrane, gathering a more representative sample of cells.
The cells are transferred to a clear glass microscope slide that is bar-coded for identification purposes. Alcohol fixative, supplied in the kit, is poured over the slide, which is then sent to a designated commercial laboratory. In addition to being reviewed by a cytopathologist, the specimen is initially digitally imaged and scanned for atypical cells by a neural net computer.
As with exfoliative cytology, the manual collection, transfer, and preservation of the cells could result in a non-representative sample. Also, extraneous material and overlapped cells may be spread on the slide. Subsequent studies have reported a number of false-positive and/or false negatives.7-9 Brush biopsy, therefore, also has a significant number of false-positives.
LIQUID-BASED BRUSH CYTOLOGY
Liquid-based brush cytology advances the effort to collect a technologically superior cell specimen. As with other procedures, this process was first used for Pap smears; brushes that were developed initially for the cervix are used effectively in the oral cavity. Currently 90% of Pap smears performed in the United States use liquid-based brush cytology.10,11
By means of a nylon bristle brush, cells are harvested from all layers of the epithelium, down to the basement membrane. This brush is immersed and twirled in a container with a special liquid preservative. In addition, the brush head is detached and included in the container. The specimen is sent to an oral pathology laboratory, where a machine filters the epithelial cells from debris, and lays them in a monolayer on a glass slide. After staining and coverslipping, the slide is examined by an oral pathologist (Figure 1).
In liquid-based brush cytology, virtually all cells are obtained because the machine is retrieving them from the liquid preservative and the brush with technician assistance. Thus, there is greater cellular representation of the observed lesion. Another advantage is that the clean, monolayer preparation of the slide aids in the screening process. This improved environment for interpretation decreases false-positives and false-negatives and offers a higher degree of certainty.12
This process also offers the opportunity to perform supplementary tests on the liquid specimen, providing additional objective information for assessment. One process, currently FDA-approved for cervical specimens, is screening for human papillomavirus (HPV), specifically the 13 oncogenic high-risk types of HPV. The causal link between HPV and posterior oral cancer has recently been proven and, thus, identifying the presence or absence of HPV types in the mouth may be a critical piece of information.13 At this time, it may be used simply to flag that patient as potentially "higher risk." Although this HPV screening of oral samples is not currently part of the FDA indication statement, it is an approved off-label use.
Another possible supplemental use of liquid cytology, already in practice in Canada, is a DNA ploidy analysis, measuring normal or abnormal (ie, aneuploidy) DNA amount in cells. Aneuploidy is not absolutely linked to oral cancer, but it is another part of the risk assessment environment.
VITAL DYES/STAINS
Another method to apply in screening for oral mucosal abnormalities is the use of tolonium chloride (toluidine blue), a vital dye capable of staining the nuclear material of cells. Dysplastic and malignant cells exhibit increased DNA synthesis, a greater nuclear/cytoplasmic ratio, and their location will retain dark blue dye after the staining and decolorization process.
In this procedure, a 1% toluidine blue solution is applied to the suspicious lesion. Rinsing the area with water, then with 1% acetic acid solution, before and after applying toluidine blue is recommended to prepare the area and remove excess dye. After-wards, a visual examination reveals any points of retained intensified blue color, which need to be closely monitored or biopsied. In addition, the stain pattern may be photographed for documentation purposes (Figure 2).
This staining process is used primarily as part of the decision process to verify the initial visual assessment. Additional information and experience are needed to accurately interpret the coloration. For example, cells that are healing also exhibit increased DNA synthesis and may retain dye, while heavily keratinized areas do not stain effectively.
If a biopsy is recommended, this stain is beneficial to indicate the sampling area for the oral surgeon, either in preparation for an immediate appointment, or photographing it for a future date. If the lesion is particularly large, the stain pinpoints the most diagnostic area for a biopsy.
This procedure, and several of the methods discussed below, uses a cytoplasmic dehydration agent: diluted acetic acid. Although there are no known adverse effects if the solution is swallowed, dentists should use caution or avoid use with certain patients. This rinse should not be used with children, pregnant or lactating patients, patients with hypersensitivity or allergic reactions, patients who have problems following directions, or patients with renal or liver insufficiency. Dentists should consult the label for further information.
Toluidine blue should also be used with care. Some patients may find the taste objectionable. The dye may stain clothing or leave residual discoloration in other areas of the mouth. If the patient swallows toluidine blue, there may be a transient change in urine or feces color.
CHEMILUMINESCENCE
After thoroughly examining the oral cavity under bright incandescent light, the dentist has other illumination options to aid in gathering more information. These devices are based on different technologies; one method is a chemiluminscent light stick. While this method is not designed to replace visual inspection and identification of lesions, there is some support for its use in wider screening of asymptomatic individuals.14
This light stick contains a nontoxic, nonthermogenic solution of peroxyalate. The solution is contained in a vial within a single-use capsule. This flexible capsule is bent to break the inner vial and activate the solution; the now-illuminated capsule is inserted into a retractor. After the mouth is rinsed with a cytoplasmic dehydration agent of 1% acetic acid solution, leukoplakic lesions with dysplastic cells will appear bright white under the light (ie, acetowhite). Normal epithelium remains dark in contrast. However, some inflamed lesions that are healing may also appear bright during this process because of increased DNA activity and turnover, and should be monitored accordingly.
Chemiluminescence was initially developed for use for cervical mucosa, and its FDA approval for oral screening is predicated on that process. The FDA statement reads: "Indicated for use as an adjunct to conventional oral mucosal screening by trained healthcare providers for the identification, evaluation, and monitoring of oral mucosal abnormalities in a population at increased risk for oral cancer."
Once the atypical area has been identified, the dentist may choose to use toluidine blue staining to mark it for docu-mentation, subsequent evaluation, or for a biopsy. An adjunct FDA statement regarding the lesion-marking system indicates its use for further evaluation and monitoring of white lesions in a population at increased risk for oral cancer.
FIBER-OPTIC TRANSILLUMINATOR
Some dental practices may already employ a transilluminator—a high-output LED light source—to aid in locating crown fractures and posterior and anterior caries. Enhanced with a diffused fiber-optic light guide, this device may be beneficial in isolating and monitoring lesions.
Tissue is prepared with an acetic solution to produce the acetowhite effect, by which the refractive properties of dysplastic epithelium are changed. With the aid of this light, these acetowhite lesions appear more defined. However, as the acetowhite effect may also occur in noncancerous, healing lesions, dentists should apply the full spectrum of their knowledge and experience for subsequent evaluation and monitoring.
This technique is noninvasive and painless, although the standard precautions for the use of acetic acid should be followed. This device is not limited to a single use; therefore, a disposable plastic sleeve fits over the guide to prevent cross-contamination.
DIRECT OPTICAL FLUORESCENCE
As a result of increased DNA activity, atypical nonkeratinized squamous epithelium exhibits changes in refractive properties. Direct optical fluorescence visualization technology is another screening option that identifies these changes. In this procedure, there is no prerinse or stain. A multi-use electrical metal halide light produces a particular excitation wavelength in a 50-mm circular area, under which normal cells emit autofluorescence. When viewed through a filtering scope that eliminates other wavelengths of light, normal cells appear green, while abnormal cells will appear dark (ie, loss of fluorescence). This fluorescent effect cannot be observed with the naked eye, nor can it be accomplished with a curing light (Figure 3 and 4).
As dysplastic progression increases, fluorescence decreases. Metabolic activity, nuclear back-scattering, micro-vascularization blood absorption, and the breakdown of the collagen matrix (a prelude to the invasion of collagen cross-links) are all processes that reduce fluorescence.
Direct optical fluorescence has recently received an expanded FDA indication statement: "Intended to be used by a dentist or a healthcare provider as an adjunct to traditional oral examination by incandescent light to enhance the visualization of oral mucosal abnormalities that may not be apparent or visible to the naked eye, such as oral cancer or premalignant dysplasia."
This process has an additional benefit summarized in its FDA statement: "Further intended to be used by a surgeon to help identify diseased tissue around a clinically apparent lesion and thus aid in determining the appropriate margin for surgical incision." If an area beyond the clinical boundaries of the lesion appears dark under these conditions, the surgeon will enlarge the biopsied area.
It must be noted that some benign conditions (eg, increased vascularity) may also cause loss of fluorescence. The lesion may need to be re-evaluated under palpation or diascopic pressure to determine its status. Also, pigmented lesions, including amalgam tattoos, may appear dark under these circumstances.
This technique is noninvasive and has no contraindications. As there are no preparation solutions used for the oral tissue, there are no patient objections to taste or staining, and no negative interactions with any other dental procedures in process.
While this device has a greater up-front expense than other methods, the possibility of using it for more widespread screening may increase its usefulness in the long term for certain practices.
FUTURE TECHNOLOGIES
Saliva has been identified as an emergent diagnostic fluid—a means to monitor the health of individuals and diagnose a variety of conditions. Salivary transcriptome diagnostics may be useful in the early detection of oral cancers. In a group study, certain biomarkers were used to discriminate and predict whether the sample was from a patient with cancer or from a control group. Specificity and sensitivity of 91% in differentiating oral squamous cell carcinoma from control samples was attained.15 In the future, an easy-to-use, handheld automated system to detect salivary protein and nucleic acid targets should be commercially available.
CONCLUSION
Ideally, oral cancer screening would be routinely practiced not only in dental offices, but in general medical examinations to increase the odds of early detection for those who are not dental patients. However, in the absence of public awareness, the dentist is the one responsible for gathering all available information in the patient’s best interest. Adjunctive screening techniques offer additional opportunities to gain potentially important data under a wide variety of real-life circumstances, although there is a debate within the oral pathology community over the validity and efficacy of current methods. Randomized, consecutive-case control studies—double-blinded and placebo-controlled with surgical biopsy confirmation—are needed.
It is up to individual dentists to determine which of these procedures fits into their practice and offers the most value to the patient. As the requirement for accurate screening intensifies, these techniques will continue to be developed to advance and improve early detection and, consequently, long-term survival.
References
1. Oral Cancer. American Cancer Society. Available at: www.cancer.org/downloads/PRO/OralCancer.pdf. Accessed May 1, 2007.2. Melnikow J, Birch S. Human papillomavirus triage of atypical squamous cells of undetermined significance: cost-effective, but at what cost? J Natl Cancer Inst. 2006;98(2):82-83.
3. What are the risk factors for oral cavity and oropharyngeal cancer? American Cancer Society. 2006. Available at: www.cancer.org.docroot/CRI/content/CRI_2_4_2X_What_are_the_risk_factors_for
_oral_cavity_and_oropharyngeal_cancer_60.asp. Accessed May 1, 2007.
4. Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772-1783.
5. Stevens M. IMVS Newsletter. 2001;43:4-5.
6. From exfoliative cytology to oral brush biopsy: an advance in the early detection of oral pre-cancers and cancers. Oral Cancer Foun-dation. 2007. Available at: www.oralcancerfoundation.org/diagnosis/detailed_brush_cytology.htm. Accessed September 7, 2007.
7. Poate TW, Buchanan JA, Hodgson TA, et al. An audit of the efficacy of oral brush biopsy technique in a specialist oral medicine unit. Oral Oncol. 2004;40(8):829-834.
8. Potter TJ, Summerlin DJ, Campbell JH. Oral malignancies associated with negative transepithelial brush biopsy. J Oral Maxillofacial Surg. 2003;61(6): 674-677.
9. Rick GM. Oral brush biopsy: the problem of false positives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(3):252.
10. Hoelund B. Implementation of liquid-based cytology in the screening programme against cervical cancer in the County of Funen, Denmark, and status for the first year. Cytopathology. 2003;14(5):269-274.
11. Southwick K. Two roads for HPV testing, but only one is heavily traveled. Available at: www.cap.org/apps/docs/cap_today/feature_stories/1103TwoRoadsForHPV.html. Accessed September 7, 2007.
12. Schledermann D, Ejersbo D, Hoelund B. Improvement of diagnostic accuracy and screening conditions with liquid-based cytology. Diagn Cytopathol. 2006;34(11): 780-785.
13. Syrjänen S. Human papillomaviruses in head and neck carcinomas. N Engl J Med. 2007;356(19):1993-1995.
14. Epstein JB, Gorsky M, Lonky S, et al. The efficacy of oral lumenoscopy (ViziLite) in visualizing oral mucosal lesions. Spec Care Dentist. 2006;26(4): 171-174.
15. Kahn MA, Epstein J. Optimizing the oral cancer evaluation. Compend Cont Educ Dent. 2007;28(Suppl):3-10.
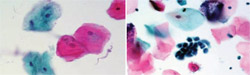 | 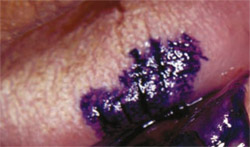 | |
| Figure 1 A liquid-based brush cytology sample. | Figure 2 Clinical example of 1% toluidine blue stain applied to a suspicious lesion for ease in visual examination. | |
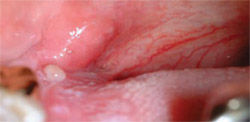 |  | |
| Figure 3 While a mild dysplasia can be seen with visible light, abnormal cells cannot be detected. | Figure 4 Under direct optical fluorescence, normal cells will appear green and abnormal cells will be dark. | |
| About the Author | ||
 Michael A. Kahn, DDS Michael A. Kahn, DDS Michael A. Kahn, DDS Professor and Chairman Department of Oral and Maxillofacial Pathology Tufts University School of Dental Medicine Boston, Massachusetts | ||



