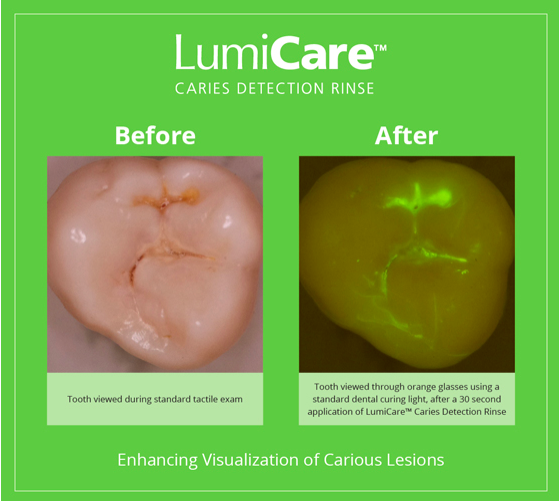
Dental Caries, the world's most prevalent chronic disease, affects more than 95% of Americans over their lifetimes and when left untreated, can lead to dental cavities. GreenMark's LumiCare™ rinse enhances visualization of carious lesions (cavities & pre-cavities) by targeting and illuminating sub-surface porosities in enamel, thereby aiding in the detection of caries. The technology utilizes bioresorbable starch-based nanoparticles which degrade due to enzymes in saliva by the time the patient is ready to leave the dental office.
Validation studies have been performed on a range of caries severity, including cavitated as well as early, non-cavitated lesions. "Since 2015, we've worked alongside our research partners at the University of Michigan, to realize our vision of detecting the early stages of caries and repairing teeth non-invasively," said Dr. Nathan Jones, M.Sc., Ph.D., co-inventor and GreenMark Vice President Technology. Identifying lesions early creates an opportunity to avoid invasive treatment options required once a cavity has formed.
"While detecting and treating caries non-invasively with this breakthrough nanotechnology sounds like science fiction, the reality is that integration into dental practices is just around the corner," said Dr. Lou Shuman, DMD, CAGS, dental technology thought leader, CEO of Cellerant Consulting Group, and founder of the Cellerant Best of Class Technology Awards. LumiCare™ rinse was recognized as a 2020 Best of Class winner in the Emerging Technology category.
Prior to launch, GreenMark will scale its manufacturing and solidify its distribution channels. "Our company's passion for creating new products that benefit dental professionals and their patients takes another step forward as we approach commercial availability of our LumiCare™ product," noted Adam Laird, J.D., Director of Business Development.
GreenMark will continue to sponsor research studies with leading clinicians and academicians on the LumiCare™ device, as well as other applications of its nanotechnology platform for non-invasive and preventative dental treatment. "Our company aims to help advance the standard of care in the management of dental caries through the contemporary scientific understanding of this disease," said Dr. Wendy Bloembergen, MD, GreenMark Vice President Clinical Affairs. "We recognize the impact that early-stage caries detection combined with minimally invasive preventative treatment can have for dental practices and their patients."
About GreenMark Biomedical Inc.
GreenMark develops products that involve small sub-micron particles produced from food grade starch. These particles make an ideal carrier for medical and dental applications, given that enzymes in our body and saliva degrade starch. Dental caries is the most prevalent chronic disease in the world, and GreenMark is developing ways to identify and better assess the disease in its early stages, monitor progression and to treat it noninvasively or non-surgically. The Company's LumiCare™ Caries Detection Rinse, to be used by dental professionals as part of the routine dental exam, contains fluorescently labeled starch particles that target the subsurface of carious lesions in enamel and illuminates them using a blue curing light. Identification at stages before cavitation will allow the use of non-surgical management options, resulting in less discomfort and improved long-term oral health outcomes for patients. GreenMark's team has also demonstrated the ability to load the essential minerals, depleted as a result of tooth decay, directly inside the small starch particles. Unlike most fluoride products, which seal the tooth's enamel surface, GreenMark's products are designed to target and restore the enamel subsurface by regenerating tooth structure.
GreenMark Biomedical Inc. has an office located at 325 E. Grand River Avenue, Suite 314, East Lansing, MI 48823 and offices & lab facilities at 1600 Huron Parkway, Building 520, 2nd Floor, Ann Arbor, MI 48109. Contact: info@greenmark.bio or (517) 896-3665. For more information, visit www.greenmark.bio.



 Today, Anatotemp is announcing the incorporation of the Anatotemp SC anatomic dental implant healing abutment and scan body library into the 3Shape dental implant laboratory and model creator software.
Today, Anatotemp is announcing the incorporation of the Anatotemp SC anatomic dental implant healing abutment and scan body library into the 3Shape dental implant laboratory and model creator software.
 In honor of World Health Day, SmileDirectClub, Inc. (Nasdaq: SDC), the next generation oral care company with the first medtech platform for teeth straightening, announced today its new Lifetime Smile Guarantee™, an industry-first offering that guarantees its customers a straighter smile for life. As a leader in oral care innovation, SmileDirectClub is committed to helping customers achieve a healthier smile, which in turn leads to improved overall health.
In honor of World Health Day, SmileDirectClub, Inc. (Nasdaq: SDC), the next generation oral care company with the first medtech platform for teeth straightening, announced today its new Lifetime Smile Guarantee™, an industry-first offering that guarantees its customers a straighter smile for life. As a leader in oral care innovation, SmileDirectClub is committed to helping customers achieve a healthier smile, which in turn leads to improved overall health.

 Ivoclar Vivadent has announced that they will be taking to the North American highways in 2021, embarking on an educational experience like no other: The 2021 Dental Innovation Tour.
Ivoclar Vivadent has announced that they will be taking to the North American highways in 2021, embarking on an educational experience like no other: The 2021 Dental Innovation Tour.
 Every four seconds, somewhere in the world, a restoration made with Ivoclar Vivadent pressed ceramics is placed. Despite the arrival of other new fabrication methods, dentists and dental technicians continue to trust this unique procedure—using it with enthusiasm and conviction. What is the secret of the sustained success of this technique? Ivoclar Vivadent takes a look at its remarkable history.
Every four seconds, somewhere in the world, a restoration made with Ivoclar Vivadent pressed ceramics is placed. Despite the arrival of other new fabrication methods, dentists and dental technicians continue to trust this unique procedure—using it with enthusiasm and conviction. What is the secret of the sustained success of this technique? Ivoclar Vivadent takes a look at its remarkable history. Vista Apex's PinkWave™ curing light is taking the dental world and the "city of festivals" by storm. Milwaukee, Wi, is lighting the Hoan Bridge pink to recognize local innovators, Vista Apex, on April 1, 2021.
Vista Apex's PinkWave™ curing light is taking the dental world and the "city of festivals" by storm. Milwaukee, Wi, is lighting the Hoan Bridge pink to recognize local innovators, Vista Apex, on April 1, 2021.
