Henry Schein Dental, the dental business of Henry Schein, Inc. (Nasdaq: HSIC) today announced the 2021 schedule for its new webinar series, ‘Breaking Down Barriers to Care: Treating Patients with Disabilities.’ The free webinar series was designed to equip oral health professionals with education and resources to help them confidently accept and effectively treat patients with disabilities. Dr. Maureen Munnelly Perry, professor of special care dentistry and the director of the Center for Advanced Oral Health at ATSU Arizona School of Dentistry & Oral Health in Mesa, AZ, is the host of this series. To register or to access on-demand, practitioners can visit,https://henryscheindigital.com/breaking-down-barriers.
According to Centers for Disease Control and Prevention, nearly one in five people has a disability in the United States, representing a significant number who may struggle with physical, intellectual, psychiatric, visual, hearing, or neurological challenges. Providing care to these patients is critical. To reinforce the Company’s commitment to helping dental practitioners have the tools to deliver critical oral care, as well as education that will help customers feel comfortable treating this population, the series addresses common disabilities, effective adaptations for treating patients, behavior management techniques, and more.
“At Henry Schein, we deliver solutions our customers can rely on to help deliver optimal care to patients,” said Jonathan Koch, Senior Vice President and CEO, Global Dental Group. “We are steadfast in our commitment to enhancing health equities among communities most in need, while helping to provide our customers with the education and know-how that will help fulfill patient’s unique oral health care needs.”
The 2021 schedule includes:
February 18 at 6:00 p.m. EDT – “Dental Considerations for Patients with Epilepsy & Seizures”
March 23 at 6:00 p.m. EDT – “Dental Discussion on Intellectual Disabilities Featuring Down Syndrome”
April 21 at 6:00 p.m. EDT – “Autism: What’s New, What’s True & How Do We Provide Care?”
Each webinar in the series is free to attend, however, for those interested to receive Continuing Education credits, 1 CE credit is available per webinar, at the cost of $20/webinar.
Henry Schein Dental first launched the series in November 2020, which has addressed a variety of topics including the current state, and barriers, in special care dentistry; the social and historical context of developmental disabilities in America; and dental management of patients with cerebral palsy and other neuromuscular disorders.
To view past webinars or to register for upcoming webinars, please visithttps://henryscheindigital.com/breaking-down-barriers.
About Henry Schein, Inc.
Henry Schein, Inc. (Nasdaq: HSIC) is a solutions company for health care professionals powered by a network of people and technology. With more than 19,000 Team Schein Members worldwide, the Company's network of trusted advisors provides more than 1 million customers globally with more than 300 valued solutions that help improve operational success and clinical outcomes. Our Business, Clinical, Technology, and Supply Chain solutions help office-based dental and medical practitioners work more efficiently so they can help provide quality care more effectively. These solutions also support dental laboratories, government and institutional healthcare clinics, as well as other alternate care sites.
Henry Schein operates through a centralized and automated distribution network, with a selection of more than 120,000 branded products and Henry Schein private-brand products in stock, as well as more than 180,000 additional products available as special-order items.
A FORTUNE 500 Company and a member of the S&P 500® index, Henry Schein is headquartered in Melville, N.Y., and has operations or affiliates in 31 countries. The Company's sales from continuing operations reached $10.0 billion in 2019, and have grown at a compound annual rate of approximately 13 percent since Henry Schein became a public company in 1995.
For more information, visit Henry Schein at www.henryschein.com, Facebook.com/HenrySchein, and @HenrySchein on Twitter.



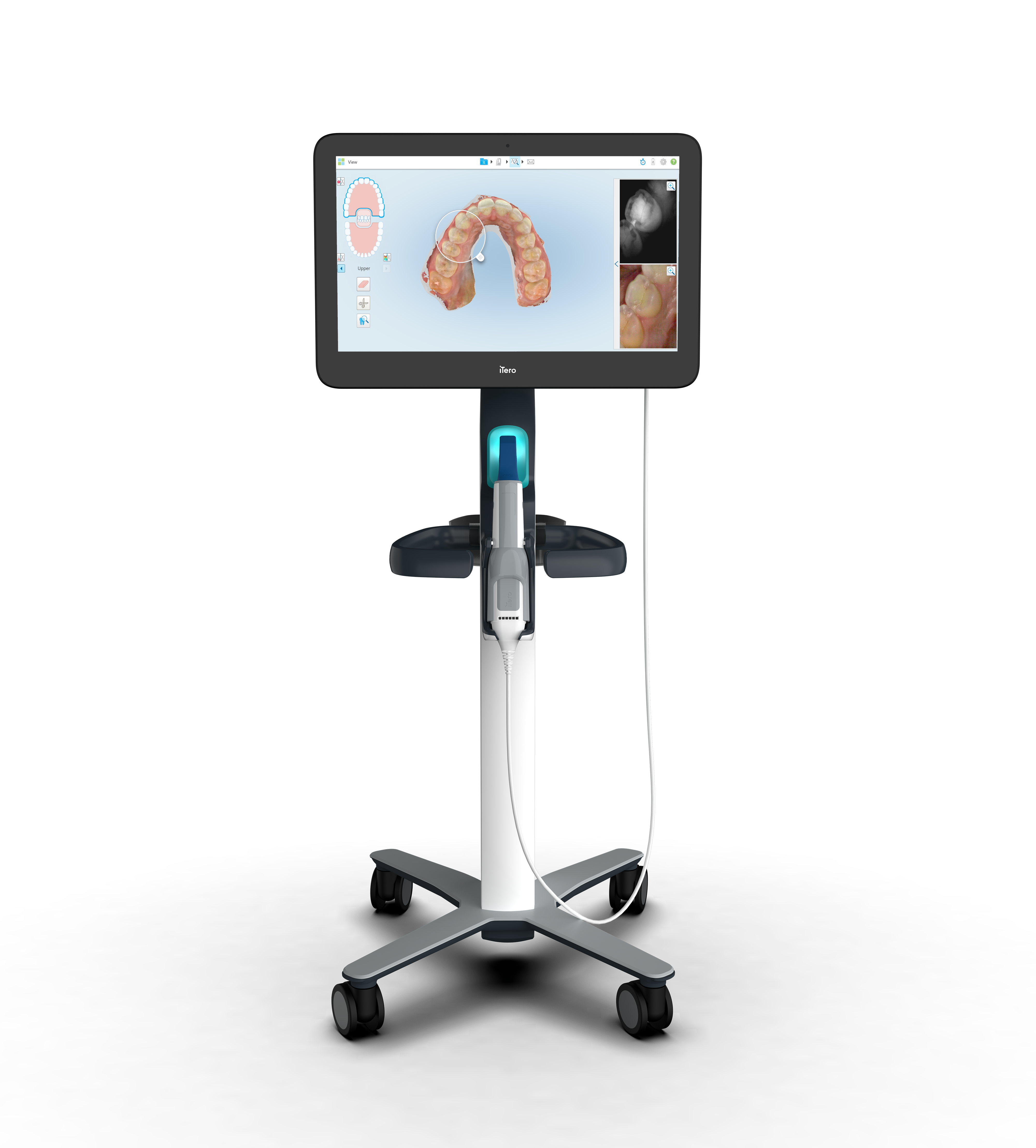 Align Technology, Inc. today announced the availability of the iTero Element® Plus Series, which expands the company’s portfolio of iTero Element scanners and imaging systems to include new solutions that serve a broader range of the dental market.
Align Technology, Inc. today announced the availability of the iTero Element® Plus Series, which expands the company’s portfolio of iTero Element scanners and imaging systems to include new solutions that serve a broader range of the dental market.
 Tokuyama Dental America is proud to announce the launch of OMNICHROMA FLOW. Following in the footsteps of OMNICHROMA, OMNICHROMA FLOW is a one-shade flowable composite, capable of matching any of the 16 Vita Classical tooth shades with a single shade of composite. By eliminating the need to shade match or stock multiple shades of composites, OMNICHROMA FLOW simplifies restorative procedures and allows dentists to reduce their inventory, saving them time and cost. OMNICHROMA FLOW is available through authorized Tokuyama Dental dealers starting February 1, 2021.
Tokuyama Dental America is proud to announce the launch of OMNICHROMA FLOW. Following in the footsteps of OMNICHROMA, OMNICHROMA FLOW is a one-shade flowable composite, capable of matching any of the 16 Vita Classical tooth shades with a single shade of composite. By eliminating the need to shade match or stock multiple shades of composites, OMNICHROMA FLOW simplifies restorative procedures and allows dentists to reduce their inventory, saving them time and cost. OMNICHROMA FLOW is available through authorized Tokuyama Dental dealers starting February 1, 2021.

 As many nonprofit dental clinics are struggling to get caught up after the COVID-19 shutdowns, and families across the country delay essential dental treatment due to financial difficulties, America’s ToothFairy has responded with a new campaign to help “Bring the Smiles Back.”
As many nonprofit dental clinics are struggling to get caught up after the COVID-19 shutdowns, and families across the country delay essential dental treatment due to financial difficulties, America’s ToothFairy has responded with a new campaign to help “Bring the Smiles Back.”
 An objective clinical study conducted by the Dental Advisor with 30 clinical evaluators and 787 total uses showed that FIT SA, the bioactive self-adhesive flowable composite from Shofu Dental Corporation, received a 92% rating (4.5+). As a result of which, 97% of the consultants would recommend this product to a colleague.
An objective clinical study conducted by the Dental Advisor with 30 clinical evaluators and 787 total uses showed that FIT SA, the bioactive self-adhesive flowable composite from Shofu Dental Corporation, received a 92% rating (4.5+). As a result of which, 97% of the consultants would recommend this product to a colleague. Today, Anatotemp is announcing the incorporation of the Anatotemp SC anatomic dental implant healing abutment and scan body library into the Exocad dental implant laboratory and model creator software. Anatotemp has become the off-the-shelf solution for creating ideal gingival emergence profile and now its second generation Anatotemp SC is both an anatomic healing abutment and digital scan body in one component. By utilizing the Anatotemp SC, there is no need to remove the healing abutment and place an impression post or scan body. Anatotemp SC performs both functions saving the implant clinician four steps and at least one appointment.
Today, Anatotemp is announcing the incorporation of the Anatotemp SC anatomic dental implant healing abutment and scan body library into the Exocad dental implant laboratory and model creator software. Anatotemp has become the off-the-shelf solution for creating ideal gingival emergence profile and now its second generation Anatotemp SC is both an anatomic healing abutment and digital scan body in one component. By utilizing the Anatotemp SC, there is no need to remove the healing abutment and place an impression post or scan body. Anatotemp SC performs both functions saving the implant clinician four steps and at least one appointment.
