On March 4, 2020, the Governor proclaimed a State of Emergency to exist in California as a result of the impacts of COVID-19 to make additional resources available, formalize emergency actions already underway across multiple state agencies and departments, and help the state prepare to respond to an increasing number of individuals requiring medical care and hospitalization as a result of a broader spread of COVID-19.
Pursuant to the Governor’s Executive Order N-39-20, during the State of Emergency, the Director of the California Department of Consumer Affairs may waive any statutory or regulatory professional licensing requirements and amend scopes of practice pertaining to individuals licensed pursuant to Division 2 of the Business and Professions Code, including dentists.
California dentists have a broad scope of practice. Business and Professions Code section 1625 provides that dentists may diagnose or treat, by surgery or other method, diseases and lesions, and they can correct malpositions of the human teeth, alveolar process, gums, jaws, or associated structures. Such diagnosis or treatment may include all necessary related procedures as well as the use of drugs, anesthetic agents, and physical evaluation.
Pursuant to Executive Order N-39-20, the Director waives Business and Professions Code section 1625 to the extent it prohibits licensed dentists from independently initiating and administering COVID-19 vaccines that are approved or authorized by the federal Food and Drug Administration (FDA) to persons 16 years of age or older and, in cases involving a severe allergic reaction, epinephrine or diphenhydramine by injection, subject to the following conditions:
• The dentist successfully completes all of the following COVID-19 training programs available through the Centers for Disease Control and
Prevention:
o “COVID-19 Vaccine Training: General Overview of Immunization Best Practices for Healthcare Providers”;
o “What Every Clinician Should Know about COVID-19 Vaccine Safety”;
o “What Clinicians Need to Know About the Pfizer-BioNTech and Moderna COVID-19 Vaccines”; and,
o “Pfizer-BioNTech COVID-19 Vaccine: What Healthcare Professionals Need to Know”.
• The dentist complies with all applicable federal and state recordkeeping and reporting requirements, including providing documentation to the patient’s primary care provider, as applicable, and enters information in the appropriate immunization registry designated by the immunization branch of the State Department of Public Health.
• The subject vaccine is administered in accordance with any applicable FDA emergency use authorization. Dentists acting within the scope of this Order may independently initiate and administer to persons 16 years of age or older any COVID-19 vaccines that are approved or authorized by the FDA, as specified, and may also initiate and administer epinephrine or diphenhydramine by injection for the treatment of a
severe allergic reaction. This order is effective immediately but may be amended as circumstances require.





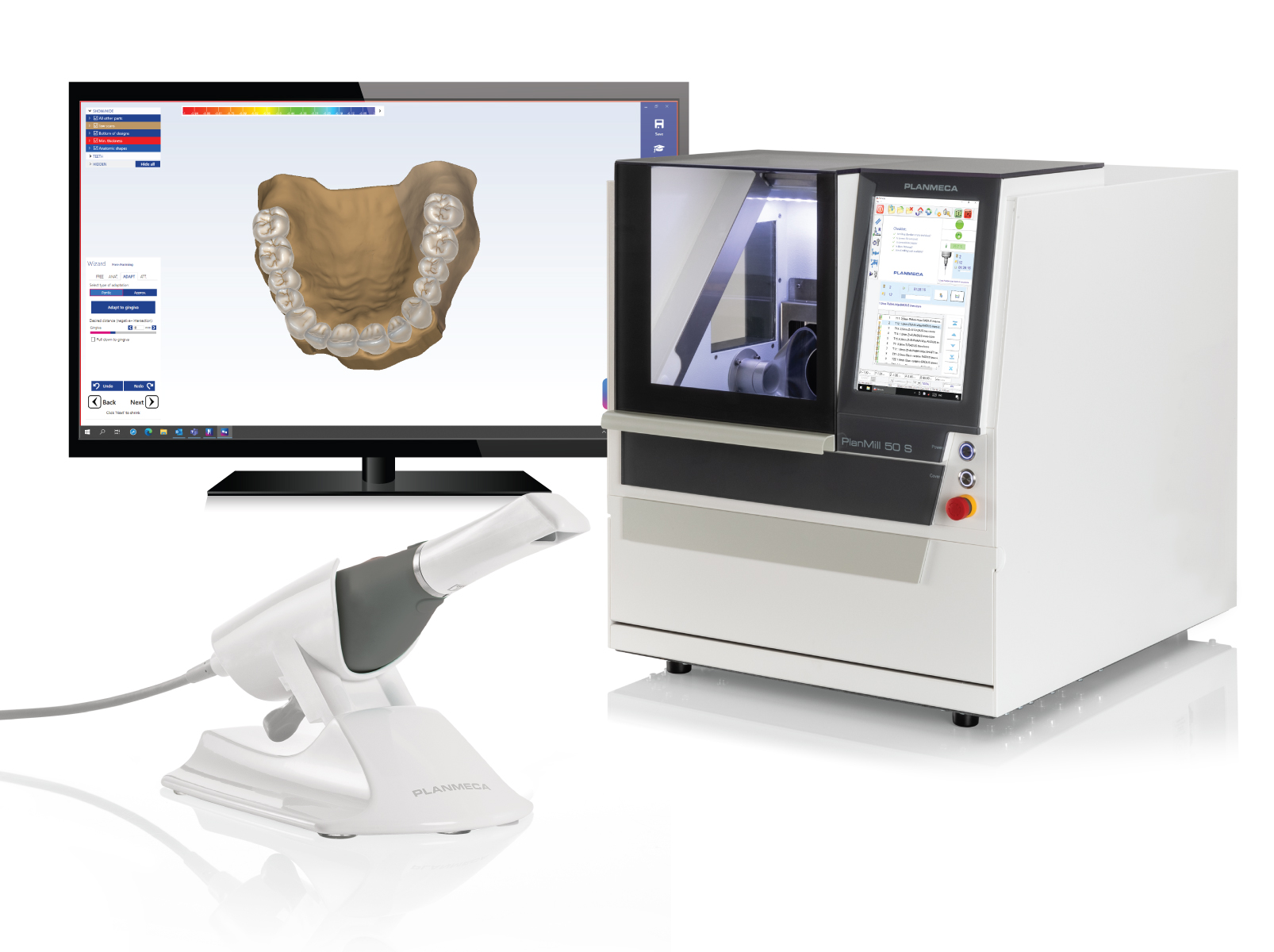 Planmeca USA, a leading manufacturer of dental imaging and CAD/CAM equipment, today announced the launch of two new CAD/CAM products – Planmeca PlanMill® 50 S 5-axis mill and Planmeca PlanCAD® Premium design software for creating dental prosthetics. These products, together with the Planmeca Emerald™ S intraoral scanner, provide a new premium solution, the Planmeca FIT® Plus. This complete solution allows clinicians to achieve maximum productivity throughout the entire digital dentistry workflow.
Planmeca USA, a leading manufacturer of dental imaging and CAD/CAM equipment, today announced the launch of two new CAD/CAM products – Planmeca PlanMill® 50 S 5-axis mill and Planmeca PlanCAD® Premium design software for creating dental prosthetics. These products, together with the Planmeca Emerald™ S intraoral scanner, provide a new premium solution, the Planmeca FIT® Plus. This complete solution allows clinicians to achieve maximum productivity throughout the entire digital dentistry workflow.


