Align Technology, Inc. (NASDAQ: ALGN) today announced that the U.S. Food and Drug Administration (FDA) has cleared Invisalign treatment with mandibular advancement for commercial availability in the U.S. As previously announced on March 5, 2017, Invisalign treatment with mandibular advancement is the first clear aligner solution for Class II correction in growing tween and teen patients that combines the benefits of the most advanced clear aligner system in the world with features for moving the lower jaw forward while simultaneously aligning the teeth. This new solution for Invisalign doctors offers a simpler, more efficient and patient-friendly treatment option than functional appliances typically used to treat teen Class II patients.
“Class II malocclusion is the largest opportunity in the teen segment, representing close to 45% of the 9 million teen case starts worldwide each year,” said Raphael Pascaud, Align Technology chief marketing officer. “We believe that Invisalign treatment with mandibular advancement offers significant benefits to both doctors and their patients and is an effective alternative for Class II elastics and other traditional functional appliances like twin blocks”.
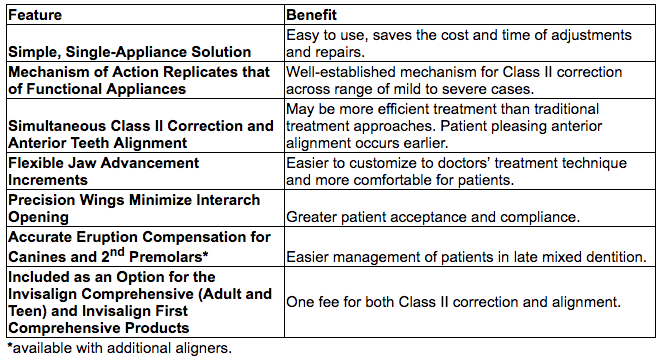
In Class II treatments, doctors align the teeth and advance the lower jaw to improve the patient’s profile. Invisalign treatment with mandibular advancement is designed to replicate the action of commonly used functional appliances for Class II correction through new “precision wings” which hold the mandible in a forward position, while simultaneously correcting malocclusion and crowding issues. Traditionally, doctors often correct the mandible position and straighten the teeth in separate phases, resulting in a longer overall treatment time.
“I think Invisalign treatment with mandibular advancement targets every deficiency we have with the old functional system,” said Dr. Sam Daher, an orthodontist with practices in Vancouver, British Columbia. “I like the fact that we can start Invisalign treatment early— we don’t have to wait until the adult teeth are in completely. We target kids right at the beginning of the growth spurt, between the ages of 11 and 12. We’re getting excellent results with these kids. I really think it’s because kids are motivated to wear their aligners to get rid of their overbites. The results really are impeccable. From an orthodontist’s point of view, it’s heaven-sent.”
Invisalign treatment with mandibular advancement can replace Class II elastics and functional appliances while offering significant benefits to both the doctor and the patient:
Invisalign treatment with mandibular advancement will be commercially available in the United States on November19, and is currently available in the following countries: Thailand, Malaysia, India, Macao, Singapore, Taiwan, Hong Kong, New Zealand, Japan, Australia, China, Malta, Hungary, Lebanon, Denmark, Ukraine, Latvia, Kuwait, Bulgaria, Guernsey, Luxembourg, Lithuania, Slovenia, Qatar, Morocco, Cyprus, Finland, Israel, United Arab Emirates, Belgium, Slovakia, Czech Republic, Greece, Poland, Portugal, Austria, Switzerland, Netherlands, Ireland, United Kingdom, Italy, Germany, Spain, France, Canada, Uruguay, Panama, Costa Rica, Chile, Colombia, Argentina, Mexico, and Brazil.
About Align Technology, Inc.
Align Technology designs and manufactures the Invisalign® system, the most advanced clear aligner system in the world, and iTero® intraoral scanners and services. Align’s products help dental professionals achieve the clinical results they expect and deliver effective, cutting-edge dental options to their patients. Visit www.aligntech.com for more information.
For additional information about the Invisalign system or to find an Invisalign doctor in your area, please visit www.invisalign.com. For additional information about iTero digital scanning system, please visit www.itero.com.