A new High Point University Poll, conducted in honor of Oral Cancer Awareness Month in April and in conjunction with the Workman School of Dental Medicine, finds a majority (60%) of North Carolina residents have not had a dental exam for oral cancer. Another one-third (33%) of poll respondents said that they have been examined.
Three-quarters (75%) of North Carolinians said they were not aware April is Oral Cancer Awareness Month, while 20% said they were aware.
The HPU Poll also asked how long it has been since they last visited a dentist or a dental clinic for any reason. Nearly half (49%) said it had been between one to 12 months, another 16% said one to two years, 16% said two to five years and 14% said it had been five or more years.
Conversations with Your Dentist
More than half of North Carolina residents are not having direct conversations with a dental professional about several important oral issues. Most poll respondents said they have not had a conversation with a dentist, a hygienist or another dental professional in the past year about how mouth diseases can include allergies and autoimmune conditions and be related to inflammatory bowel disorders (71%) or that the human papilloma virus (HPV) infection can cause oropharyngeal cancer (71%).
Majorities said they have not had a conversation with a dental professional in the past year about the dental health benefits of checking their blood sugar (70%), how diseases of the teeth, gums and mouth are related to conditions such as diabetes, heart disease and Alzheimer’s disease (67%), the benefits of giving up cigarettes or other types of tobacco to improve their dental health (64%), how mouth diseases can be related to underlying nutritional deficiencies and food intolerances (64%), and the importance of examining their mouths for oral cancer (59%).
Oral Cancer Risk Factors
A majority (68%) of poll respondents said they were not aware HPV infection is the main risk factor for oropharyngeal cancer. Only about one-quarter (26%) said they were aware of this risk factor.
Asked if they were aware of any other risk factors for oral cancer, most (81%) said they were aware of tobacco and smokeless tobacco being a risk factor for oral cancer. A majority of these same respondents were also aware that inflammation (57%), alcohol consumption (55%), precancerous lesions (54%) and genetic predisposition (50%) were risk factors for oral cancer. Less than half were aware that HPV infection (48%), oral microbiome (40%) and field cancerization (29%) were risk factors.
Brushing Teeth, Tooth Decay and Toothaches
When asked about using dental floss or other devices to clean between their teeth, about one-quarter (24%) of North Carolinians said they flossed every day, and 19% said they didn’t floss at all in the past week.
When North Carolinians were asked how many of their permanent teeth have been removed because of tooth decay or gum disease, almost half (44%) said none of their teeth had been removed because of tooth decay or gum disease. About one-third (30%) said between one and five teeth have been removed, 12% said six or more but not all and 10% said all of their teeth had been removed.
About one-third (34%) of respondents said in the past year they have never had painful aches anywhere in their mouth. Another one-quarter (27%) said they hardly ever had such pain, while 20% said occasionally and 10% said fairly often. Only 7% said they very often have painful aches anywhere in their mouth in the past year.
“This survey highlights the need for the entire health care community to remove silos and be at the forefront of educating patients on the risk factors associated with oral cancer,” says Dr. Ali Shazib, dean of HPU’s Workman School of Dental Medicine. “A systematic oral cavity examination with proper patient education performed annually would bring us one step closer to early detection and better outcomes. The time is now for us to unite to have those important conversations.”
HPU Poll 102 was fielded by the High Point University Survey Research Center on March 22 through March 30 as an online survey using a panel of respondents recruited and maintained by Dynata. Dynata sent invitations to its panel of N.C. respondents and the SRC collected 1,016 total responses on its Qualtrics platform. The items reported in this release were presented to one-third of the total respondents for an effect sample size of 347. The SRC verified that the demographics of this sub-sample were similar to those of the full sample. The SRC did all data analysis. The online sample is from a panel of respondents, and their participation does not adhere to usual assumptions associated with random selection. Therefore, it is not appropriate to assign a classic margin of sampling error for the results. In this case, the SRC provides a credibility interval of plus or minus 3.4 percentage points to account for a traditional 95% confidence interval for the estimates (plus or minus 3.1 percentage points) and a design effect of 1.2 (based on the weighting). For the sub-sample, the credibility interval is plus or minus 5.8 percentage points (5.3 adjusted to account for a design effect of 1.2 based on the weighting) The data is weighted toward population estimates for age, gender, race, ethnicity, and education based on U.S. Census numbers for North Carolina. Factors such as question wording and other methodological choices in conducting survey research can introduce additional errors into the findings of opinion polls. Percentages may not add to 100 because of rounding.
Further results and methodological details from the most recent survey and past surveys can be found at the Survey Research Center website. The materials online include past press releases as well as memos summarizing the findings (including approval ratings) for each poll since 2010.
The HPU Poll reports methodological details in accordance with the standards set out by AAPOR’s Transparency Initiative, and the HPU Survey Research Center is a Charter Member of the Initiative.
You can follow the HPU Poll on X.
Dr. Martin Kifer, chair and associate professor of political science, serves as the director of the HPU Poll, and Brian McDonald is the associate director of the HPU Poll.
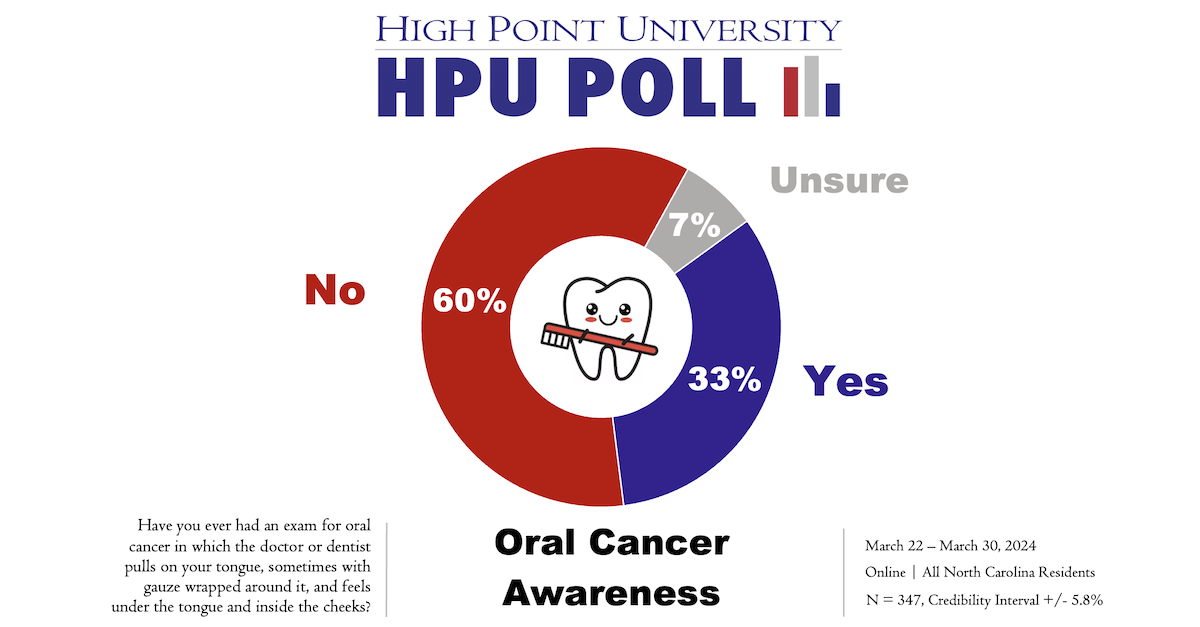
A new High Point University Poll, conducted in honor of Oral Cancer Awareness Month in April and in conjunction with the Workman School of Dental Medicine, finds a majority (60%) of North Carolina residents have not had a dental exam for oral cancer. Another one-third (33%) of poll respondents said that they have been examined.





 Founded by a team of industry pioneers, OrthoFX™, announced the new generation of advanced clear aligner polymer, NiTime™ Clear Aligners. NiTime is the first and only clear aligner system explicitly designed for shorter wear time and cleared by the U.S Food and Drug Administration (FDA) 510(k), and is now available nationwide for doctors to treat all classes of dental malocclusions. OrthoFX will showcase the new breakthrough material at the AAO (American Association of Orthodontists) Annual Meeting in New Orleans, May 3-6, 2024.
Founded by a team of industry pioneers, OrthoFX™, announced the new generation of advanced clear aligner polymer, NiTime™ Clear Aligners. NiTime is the first and only clear aligner system explicitly designed for shorter wear time and cleared by the U.S Food and Drug Administration (FDA) 510(k), and is now available nationwide for doctors to treat all classes of dental malocclusions. OrthoFX will showcase the new breakthrough material at the AAO (American Association of Orthodontists) Annual Meeting in New Orleans, May 3-6, 2024.
 3Shape launches Unite 3rd Generation, allowing dental professionals to access and work with their digital impressions and patient cases anywhere, anytime, via any mobile, tablet (Android, iOS), or web device. This update enables seamless and secure cloud storage for all 3Shape TRIOS intraoral scans and patient data.
3Shape launches Unite 3rd Generation, allowing dental professionals to access and work with their digital impressions and patient cases anywhere, anytime, via any mobile, tablet (Android, iOS), or web device. This update enables seamless and secure cloud storage for all 3Shape TRIOS intraoral scans and patient data.
 Synchrony (NYSE: SYF), a consumer financial services company, today announced a multi-year renewal with
Synchrony (NYSE: SYF), a consumer financial services company, today announced a multi-year renewal with 
 Medit (
Medit (
 Delta Dental released its
Delta Dental released its  Glidewell and ProMonitoring™ announced their strategic partnership aimed at offering comprehensive clear aligner solutions tailored to the general dentist. The result is the release of the new Glidewell™ Clear Aligners: Powered by ProMonitoring.
Glidewell and ProMonitoring™ announced their strategic partnership aimed at offering comprehensive clear aligner solutions tailored to the general dentist. The result is the release of the new Glidewell™ Clear Aligners: Powered by ProMonitoring. Midmark Corp., a leading dental solutions provider focused on the design of the clinical environment to improve the delivery of care, has announced a new free goods offer for dental mechanical room solutions. Now through Nov. 27, 2024, dentists and dental facilities may select free mechanical room accessories with qualifying Midmark air compressor and vacuum purchases.
Midmark Corp., a leading dental solutions provider focused on the design of the clinical environment to improve the delivery of care, has announced a new free goods offer for dental mechanical room solutions. Now through Nov. 27, 2024, dentists and dental facilities may select free mechanical room accessories with qualifying Midmark air compressor and vacuum purchases. Roland DGA’s DGSHAPE Americas dental business group has announced the addition of the 3DX Dental 3D Printer Bundle, Powered by Roland DGA to its line of dental products and accessories. This bundle offers a reliable, full-service 3D printing solution at one affordable price, along with Roland DGA’s world-renowned customer service and support. Equipped with the 3DX bundle, dental businesses can produce a wide variety of products in-house quickly, easily, and cost-effectively, including dental models, dentures, provisional crowns and bridges, occlusal guards, surgical guides, custom clear retainers, removable die fits, and more.
Roland DGA’s DGSHAPE Americas dental business group has announced the addition of the 3DX Dental 3D Printer Bundle, Powered by Roland DGA to its line of dental products and accessories. This bundle offers a reliable, full-service 3D printing solution at one affordable price, along with Roland DGA’s world-renowned customer service and support. Equipped with the 3DX bundle, dental businesses can produce a wide variety of products in-house quickly, easily, and cost-effectively, including dental models, dentures, provisional crowns and bridges, occlusal guards, surgical guides, custom clear retainers, removable die fits, and more.

