Restoring Maxillary Incisors With Lingual Pit Caries
Dens invaginatus defects complicate debridement, increase risk of pulp exposure
Theodore P. Croll, DDS | Gerald A. Ferretti, DDS, MS, MPH
The presence of lingual pits and grooves in permanent maxillary incisors presents the clinical dentist with unique challenges. The depth and anatomical complexity of such malformations can be even more significant when the tooth is affected by "dens in dente" (ie, tooth within a tooth), which is also known as dens invaginatus. According to Hülsmann, "Dens invaginatus is a developmental anomaly resulting in a deepening or invagination of the enamel organ into the dental papilla prior to calcification of the dental tissues."1 At the time of this article's writing, PubMed® searches of "dens in dente," "dens invaginatus," and "lingual pits in maxillary incisors" yielded 563, 676, and 3 articles, respectively, and a large number of all of these listings were related to endodontic therapy for affected teeth with infections.1-12
This article documents the treatment of two young patients who presented with lingual pit caries lesions in their permanent maxillary incisors: a 12-year-old girl who had single lingual pits incisal to the cingula of her two maxillary lateral incisors and an 8-year-old girl who had double pits in both of her maxillary lateral incisors, all of which were associated with dens invaginatus, and a pit in an adjacent maxillary central incisor that was also associated with dens invaginatus. To accomplish the goals of sufficient debridement of the carious enamel and dentin, reliable disinfection of the cavity preparation, pulpal healing and preservation of pulp vitality, and long-term closure and sealing off of the defect while avoiding the need for pulp extirpation and endodontic obturation, a specific, step-by-step clinical treatment protocol was followed.
Treatment Protocol
The steps in this treatment protocol for the restoration of anterior teeth with caries infected lingual pit/groove defects included the following:
1. Local infiltration of a suitable anesthetic
2. Thorough cleaning of the affected teeth with prophylaxis paste
3. High-speed, water-cooled, conservative cavity access with a suitable diamond or carbide bur
4. Slow-speed outlining of the cavity, followed by debridement of as much carious tooth substance as possible without mechanical pulp exposure (Round burs and inverted cone burs are ideal for this purpose. This step requires great care because of the proximity of the lesion to the pulp space wall and the uncertainty regarding the internal spread of the infection and the internal anatomical form of the pit/groove defect. These concerns are particularly relevant when restoring a tooth affected by dens invaginatus.)
5. Disinfection of the cavity preparation by soaking it in a 5% glutaraldehyde/35% hydroxyethyl methacrylate (HEMA) solution13,14
6. Deep interior filling of the cavity preparation using a suitable calcium silicate liner (ie, indirect pulp capping)15-19
7. Etched and adhesively bonded cavity filling, including overlapping the cavosurface margins with the filling material to cover much of the lingual fossa for an optimal long-term seal
8. Evaluation of incisal contacts in mandibular excursions and required adjustments
9. Follow-up and resealing, as needed, as time progresses20
Case Report 1
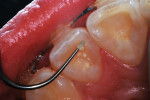
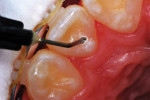
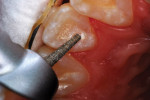
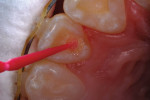
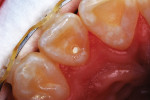
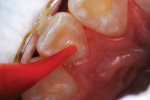
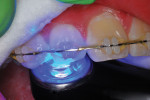
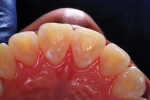
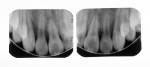
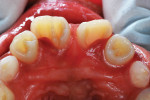
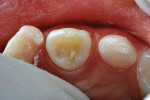
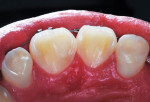
A 12-year-old girl presented with plaque-filled carious pits incisal to the cingula of her maxillary lateral incisors (Figure 1 and Figure 2). The teeth were treated one at a time. After local infiltration of an anesthetic, the right lateral incisor was cleaned with prophylaxis paste using a rubber cup and a rotating brush. A water-cooled, high-speed small round bur was then used to create the cavity access. Next, round burs and inverted cone burs were used at slow speed to shape the preparation and debride the carious dentin (Figure 3). Great care was taken to avoid pulp space perforation. To achieve disinfection of the cavity preparation, it was soaked with a 5% glutaraldehyde/35% HEMA solution (GLUMA® Desensitizer, Kulzer) for 60 seconds using a fine-tipped applicator, air-dried, soaked for another 60 seconds, and air-dried again (Figure 4).13,14 After the cavity preparation was completely air-dried, a tricalcium silicate liner (TheraCal LC®, BISCO) was carefully injected to avoid air entrapment (Figure 5). A thin condenser was then inserted to ensure that the liner was adapted into the deepest regions of the preparation. After the liner was polymerized with 10 seconds of exposure to a curing light (1,100 mW/cm2) and the excess on the cavity walls was cut away with an inverted cone bur at slow speed, a round-tipped diamond bur was used at slow speed to roughen the enamel surface in the lingual fossa peripheral to the cavity opening (Figure 6). Next, a self-etching resin bonding agent (Adper™ Prompt™, 3M) was applied to the cavity preparation and the surrounding enamel of the lingual fossa and agitated with the applicator for 30 seconds (Figure 7). After gentle air was used to blow the excess bonding agent, the curing light was applied for 10 seconds (Figure 8), and a bioactive composite restorative material (ACTIVA™ BioACTIVE - RESTORATIVE™, Pulpdent) was injected until overfill (Figure 9). A ball burnisher was then used to spread the overfilled material evenly across the lingual fossa, and it was cured using two 10-second applications of the curing light beam (Figure 10). The protocol was repeated to treat the patient's maxillary left lateral incisor. Both restored lateral incisors can be seen in a 32-month follow-up postoperative photograph (Figure 11) and radiographs (Figure 12).
Case Report 2
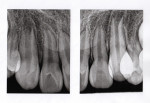
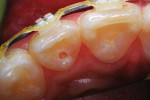
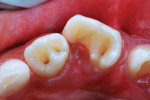
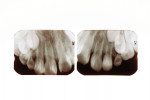
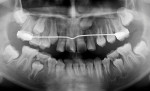
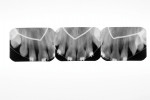
An 8-year-old girl presented for her routine 6-month recall appointment. She had been adopted from Guatemala when she was 6 months old and had an unremarkable dental history during her toddler and early elementary school years. After her permanent maxillary incisors erupted, it was observed that the lateral incisors had the appearance of mandibular premolars with biting surfaces, not incisal edges, as well as two pits in each tooth (Figure 13). In addition, her maxillary right central incisor also had a developmental pit in the mesial 1/3 of the lingual fossa (Figure 14). Preoperative radiographs confirmed the diagnoses of dens invaginatus in all three incisors (Figure 15). A cone-beam computed tomography (CBCT) imaging study was considered but not pursued because it was determined that the information would not influence the treatment plan.
The patient's orthodontist needed to refer her for surgical exposure of her mesially inclined, palatally impacted maxillary left permanent canine tooth. That tooth was exposed and moved into position with good results. To avoid any influences that might predispose the maxillary incisors to internal or external root resorptive changes, the orthodontist used extremely gentle forces on them.
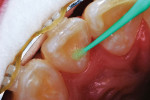
Although the restorative treatment for this child was virtually identical to that of the patient in the first case report, including local anesthetic injections, carious substance debridement, cavity preparation disinfection, tricalcium silicate liner placement (Figure 16), diamond bur roughening of the peripheral enamel, application of the self-etching resin bonding agent, overfilling of the preparation with the restorative material, spreading of the material over the peripheral enamel, and final photopolymerization, the debridement of the carious dentin was more complicated. A chief goal in both patients was to avoid devitalization of the dental pulps. Aiming to debride sufficient carious dentin by rotary instrumentation with a round bur at slow speed in teeth affected by dens invaginatus, which have characteristically complex internal pulp spaces, involves guesswork and a significant possibility of pulp space exposure. Therefore, the goal was to carefully create cavity preparations that could be reliably disinfected, internally insulated with the calcium silicate material for pulp healing and protection, and sealed, along with the lingual fossa, by an adhesively bonded restorative material.
The three restored teeth can be seen on a panoramic radiograph supplied by the patient's orthodontist 34 months after restoration (Figure 17) as well as clinically and radiographically 55 months postoperatively (Figure 18 and Figure 19).
Discussion
The two applications of glutaraldehyde/HEMA solution for disinfection and the placement of the calcium silicate liner were considered to be intrinsic to the success of the incisor lingual pit repair described. First, bacteria are eliminated or at least reduced to the point of inactivity, and then, the calcium hydroxide release supplies "a source of reparative ions, creating a sustaining alkaline environment to promote dentin tissue healing, providing immediate bond and sealing properties, and stimulating hydroxyapatite and secondary dentin formation within affected tissues."21 The acid-etched enamel bonding of the bioactive composite restorative material and the manner in which it is spread over much of the treated surface provides a truly complete seal of the preparation. The integrity of this seal should be routinely monitored at recall appointments, and if any open margins are detected, those regions can be reliably resealed.20 Such open margins have not been detected in 32 months for the patient in the first case (Figure 11) or in 55 months for the patient in the second case (Figure 18).
Acknowledgement
The authors would like to acknowledge with admiration and appreciation the unique and invaluable work of Rella Christensen, RDH, PhD, and her team at TRAC Research regarding tooth disinfection during dental restorative procedures.
About the Authors
Theodore P. Croll, DDS
Clinical Professor
Case Western Reserve University
School of Dental Medicine
Cleveland, Ohio
Adjunct Professor
University of Texas Health
Science Center at San Antonio
School of Dentistry
San Antonio, Texas
Clinic Director
Cavity Busters Doylestown
Doylestown, Pennsylvania
Gerald A. Ferretti, DDS, MS, MPH
Professor and Chair
Pediatric Dentistry Department
Case Western Reserve University
School of Dental Medicine
Cleveland, Ohio
Chief of Dentistry
The Irving and Jeanne Tapper
Dental Center
UH Rainbow Babies &
Children's Hospital
Cleveland, Ohio
References
1. Hülsmann M. Dens invaginatus: aetiology, classification, prevalence, diagnosis, and treatment considerations. Int Endod J. 1997;30(2):79-90.
2. Oehlers FA. Dens invaginatus (dilated composite odontome): I. Variations of the invagination process and associated anterior crown forms. Oral Surg Oral Med Oral Pathol. 1957;10(11):1204-1218.
3. Ulmansky M, Hermel J. Double dens in dente in a single tooth: report of a case and radiologic study of the incidence of small dens in dente. Oral Surg Oral Med Oral Pathol. 1964;17(1):92-97.
4. Conklin WW. Double bilateral dens invaginatus in the maxillary incisor region. Oral Surg Oral Med Oral Pathol.1975;39(6):949-952.
5. García-Godoy FM, Mañón Rossi WI. Familial caries distribution in human permanent teeth: lingual pits in maxillary central and lateral incisors. Acta Odontol Pediatr. 1986;7(1):7-9.
6. Hülsmann M, Hengen G. Severe dens invaginatus malformation: report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(4):456-458.
7. Alani A, Bishop K. Dens invaginitus. Part 1: classification, prevalence and aetiology. Int Endod J. 2008;41(12):1123-1136.
8. Baumgart M, Hänni S, Suter B, et al. [Dens invaginatus: review of the literature and diagnostic and therapeutic guidelines]. Schweiz Monatsschr Zahnmed. 2009;119(7):697-714.
9. Choudhari S, Joshi S, Patil N, Kalyan S. Dens invaginatus: a case report. Compend Contin Educ Dent. 2013;34(3):e53-56.
10. Hegde V, Morawala A, Gupta A, Khandwawala N. Dens in dente: A minimally invasive nonsurgical approach! J Conserv Dent. 2016;19(5):487-489.
11. Gallacher A, Ali R, Bhakta S. Dens invaginatus: diagnosis and management strategies. Br Dent J. 2016;221(7):383-387.
12. Zhu J, Wang X, Fang Y, et al. An update on the diagnosis and treatment of dens invaginatus. Aust Dent J. 2017;62(3):261-275.
13. Clinicians Report Foundation. Disinfection of tooth preparations-why and how? Clinicians Report. 2009;2(11):1-2.
14. Clinicians Report Foundation. How to predictably reduce post-op tooth sensitivity and caries. Clinicians Report. 2020;13(10):1-2.
15. Gandolfi MG, Siboni F, Taddei P, et al. Apatite-forming ability of TheraCal pulp-capping material. J Dent Res. 2011;90(special issue A):Abstract 2520.
16. Gandolfi MG, Siboni F, Prati C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J. 2012;45(6):571-579.
17. Gurcan AT, Seymen F. Clinical and radiographic evaluation of indirect pulp capping with three different materials: a 2-year follow-up study. Eur J Paediatr Dent. 2019;20(2):105-110.
18. Casamassimo PS, Fields HW, McTigue DJ, Nowak AJ. Pediatric Dentistry: Infancy Through Adolescence. 5th ed. Elsevier/Saunders; 2013.
19. Clinicians Report Foundation. Pulp capping: the viable alternative to root canal treatment. Clinicians Report. 2020;13(8):1,4-6.
20. Croll TP, Donly KJ. Resin-based composite margin repair. Inside Dentistry. 2008; 4(6);92-93.
21. Cannon M, Gerodias N, Vieira A, et al. Primate pulpal healing after exposure and TheraCal application. J Clin Pediatr Dent. 2014;38(4):333-337.