Alveolar Ridge Preservation via Socket Grafting
PLGA-coated β-TCP graft material eliminates the need for augmentation at the time of implant placement
Hanae Saito, DDS, MS
Current evidence indicates that physiologic alveolar bone resorption occurs as a consequence of tooth extraction within the first 3 months of healing. Preservation of the alveolar ridge is often necessary to permit the placement of dental implants as well as to provide favorable esthetics and successful long-term outcomes for implant restorations. Following tooth extraction, alveolar ridge preservation is indicated to prevent extensive resorption and reduce the need for ancillary ridge augmentation associated with delayed implant placement.1 Ridge preservation via socket grafting, either with or without a barrier membrane, has become a viable treatment option with some bone grafting materials. A recent randomized controlled trial found that when compared with unassisted socket healing, alveolar ridge preservation was superior in the maintenance of alveolar bone and reduced the need for additional bone augmentation at the time of implant placement.2
Although autogenous bone has historically been considered the gold standard graft material, other graft materials, such as demineralized bone allograft, deproteinized bovine bone, and alloplasts, have also shown effectiveness in ridge preservation. Beta-tricalcium phosphate (β-TCP), an alloplast, is a resorbable, osteoconductive bone substitute that supports bone formation in experimental and human clinical applications, including alveolar ridge preservation techniques. When β-TCP is coated with a biodegradable polymer, such as poly(lactic-co-glycolic acid) (PLGA), it may offer improved handling characteristics through specifically engineered properties. Chairside preparation of PLGA-coated β-TCP involves its activation by an additional agent (ie, an aqueous solution of N-methyl-2-pyrrolidone, [NMP]), which produces a sticky, moldable material. Following application, the subsequent clearance of the NMP activator results in solidification, promoting graft stabilization and particle retention in the socket and eliminating the need for coverage by a membrane in certain cases with intact socket walls.3,4 This case report describes the use of a PLGA-coated β-TCP bone substitute in alveolar ridge preservation via socket grafting.
Case Report
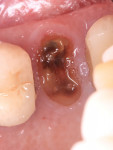
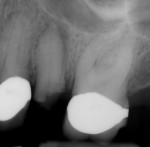
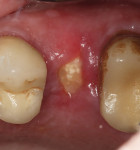
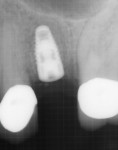
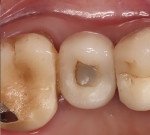
A 68-year-old female patient presented for treatment for tooth No. 13. The crown had fractured subcrestally, and the remaining tooth structure exhibited a periapical radiolucency on the pretreatment radiograph; therefore, the tooth was deemed unrestorable (Figure 1 and Figure 2). To restore function, the patient elected to receive an implant-supported restoration, and alveolar ridge preservation was chosen as part of the treatment plan. No contradictory medical or dental history was noted during the examination.
To begin, tooth No. 13 was extracted atraumatically without flap elevation. The decision to avoid flap elevation was made to optimize the remaining blood supply from the periosteum and endosteum for maximum healing potential. To achieve atraumatic removal, the roots were sectioned with a surgical length bur, then the socket was thoroughly debrided, and the integrity of the buccal plate was verified with a University of North Carolina periodontal probe. Additional barrier membrane was not needed in this case because the socket walls were intact. Use of a barrier membrane should be considered when dehiscence or fenestration is noted as well as in larger sockets to prevent partitioning of the soft tissue, which can contribute to poorer bone quality.
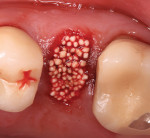
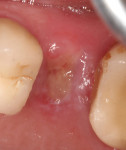
After extraction and debridement, a prefilled PLGA-coated β-TCP graft syringe (GUIDOR® easy-graft® CLASSIC, Sunstar) was prepared per the manufacturer's instructions, and the moldable graft material was then placed into the socket to the level of the crestal bone and adapted to the internal socket morphology (Figure 3 and Figure 4). Upon contact with the patient's blood, the NMP liquid activator is flushed out of the material, causing it to harden in approximately 1 minute. In order to properly deliver the granules of graft material to the apex of the socket, segmental adaptation and condensation techniques were employed. Failure to fully adapt the graft into the apical third of the socket may result in inferior bone development or complications. Once the graft material was placed, no attempt was made to adapt the wound margins or to achieve primary wound closure. Postoperative medications (ie, antibiotic, analgesic, oral antiseptic rinse) were prescribed, and the patient was scheduled for recall appointments to evaluate wound healing. The observed course of healing was uneventful. At the 7-day follow-up, slight edema and erythema were noted at the gingival margin (Figure 5); however, this had diminished by the 14-day follow-up (Figure 6). The surface of the grafted socket was completely covered by soft tissue 4 weeks after the surgery.
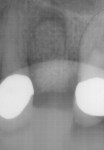
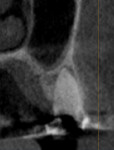
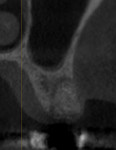
At the end of a 4-month healing period following extraction and ridge preservation, the patient returned to the clinic for implant placement. A comparison of a cone-beam computed tomography (CBCT) image taken prior to socket grafting (Figure 7) with one taken after healing but prior to implant placement (Figure 8) revealed that the alveolar ridge dimensions were adequately maintained. After the administration of local anesthesia, a full-thickness flap was elevated, and adequate preservation of the alveolar ridge to support the implant placement could be visualized. An osteotomy was created per the manufacturer's recommendations. During the preparation of the osteotomy, the patient's bone quality was determined to be D3 using the Misch classification of bone density.5 For large or poorly healing extraction sockets, a longer healing period prior to implant placement may be needed, and appropriate alterations to the implant placement protocol should be considered. In this case, the osteotomy was prepared in an undersized manner, and then a 4.3 x 8 mm tapered implant (Replace Select™ Tapered, Nobel Biocare) was placed with approximately 30 Ncm of torque (Figure 9). A cover screw was then placed, and the flap was approximated to achieve primary closure and sutured with polyglycolic acid sutures. The patient was prescribed postoperative medications and referred to the restorative dentist. Eight months after the implant placement, a screw-retained restoration was delivered (Figure 10). At a follow-up appointment 30 months after loading, the implant supported restoration was evaluated and noted to have uneventful function (Figure 11 and Figure 12).
Conclusion
Alveolar ridge preservation using an in situ-hardening PLGA-coated β-TCP bone substitute resulted in maintenance of the patient's alveolar ridge and facilitated implant placement with no need for additional bone augmentation at the time of delivery. Using this method, clinicians can expect feasible implant placement and survival.
About the Author
Hanae Saito, DDS, MS
Clinical Associate Professor
Director, Predoctoral Periodontics
Division of Periodontics
University of Maryland
School of Dentistry
Baltimore, Maryland
References
1. Avila-Ortiz, G, Chambrone L, Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: a systematic review and meta-analysis. J Clin Periodontol. 2019;46(Suppl 21):195-223.
2. Avila-Ortiz G, Grubler M, Romero-Bustillos M, et al. Efficacy of alveolar ridge preservation: a randomized controlled trial. J Dent Res. 2020;99(4):402-409.
3. Saito H, Shiau HJ, Prasad H, Reynolds MA. Evaluation of a poly(lactic-co-glycolic) acid-coated beta-tricalcium phosphate bone substitute for alveolar ridge preservation: case series. Clin Adv Periodontics. 2017;7(4):190-194.
4. Saito H, Couso-Queiruga E, Shiau HJ. et al. Evaluation of poly lactic-co-glycolic acid-coated β-tricalcium phosphate for alveolar ridge preservation: A multicenter randomized controlled trial. J Periodontol. 2020. doi:10.1002/JPER.20-0360.
5. Misch CE, Hoar J, Beck G, et al. A bone quality-based implant system: a preliminary report of stage I & stage II. Implant Dent. 1998;7(1):35-42.
MANUFACTURER INFORMATION
Sunstar
us-professional.gumbrand.com/guidor
877-484-3671