32-Year Follow-up of a Class IV Central Incisor Restoration
A case report and resin-based composite retrospective
Theodore P. Croll, DDS | Steven R. Jefferies, MS, DDS, PhD
Often times articles in the dental literature detail step-by-step clinical procedures and depict the end result without showing any long-term follow-up, or perhaps present only 6 to 12 months of photographic documentation. The least informative of such reports are those that provide an immediate postoperative view but no follow-up images. A case report or clinical technique article that shows the results of treatment over many years can be a valuable contribution to the dental profession. This report documents a Class IV resin-based composite (RBC) restoration of a fractured central incisor in a 10-year-old girl, with photographs recorded at 2 years, 5.5 years, and 32 years after treatment. In addition, re-repair of the tooth is featured 32 years after initial repair.
Case Report
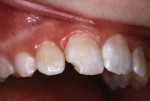
In 1983, a 10-year-old girl sustained a Class IV disto-incisal corner fracture of her maxillary right central incisor (Figure 1). The patient struck the tooth on a brick wall at her school playground and was brought to the office for emergency treatment.
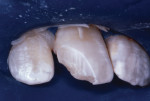
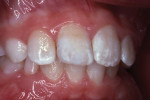
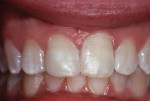
The tooth was not mobile and a radiograph confirmed that the root was neither fractured nor displaced. The coronal fracture penetrated into the dentin, but there was no pulp space exposure. A diagonal internal craze fracture across the crown was noted several millimeters from the detached fracture, extending from the mesio-incisal angle to midway up the distal surface (Figure 1). Exposed dentin was covered with Dycal® (DENTSPLY, www.dentsplysirona.com) calcium hydroxide liner, and the fracture site was repaired with an acid-etch–retained RBC insulating protective “bandage.”1 Two months later, the tooth was restored for the long term with Heliosit (Ivoclar Vivadent [originally Vivadent], www.ivoclarvivadent.com), a composite used in that time period (Figure 2). Helioseal (Vivadent), a bright white resin sealant, was applied as a surface coloration agent, to simulate the patient’s idiopathic generalized white enamel “dysmineralization”2 discoloration (Figure 3 and Figure 4). Both of these products were based on a UDMA (urethane dimethacrylate) formula, not BIS-GMA (bisphenol A glycidyl methacrylate).
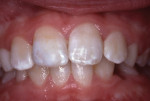
A report of this tooth repair was published in 1984.3 The tooth was photographed again in 1988, 5.5 years after the injury and repair (Figure 5). The patient, then aged 16 years, was transferred by her parents to a family dental office for routine adult care.
In 2015, the patient’s younger sister, who was in the senior author’s office with her 2-year-old child, reported that her older sister’s “front-tooth filling had discolored.” She also provided her sister’s phone number and related that she still lived in the area. The patient from 1983 was called and an appointment made to evaluate her, 32 years after treatment of the central incisor.
32 Years Later
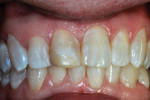
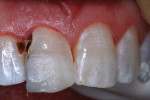
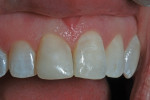
The patient, who was now aged 42 years, presented with the original restoration structurally intact. However, the white enamel surface sealant was absent, the RBC had turned yellow-brown, and resin/enamel margins had slight separation and some stain (Figure 6). No pathological changes were seen on a periapical radiograph except for a Class III caries lesion of the distal surface of the central incisor. The patient reported no discomfort and said there had been no need for additional treatment over the years, but she voiced displeasure with the color of the tooth. The clinician performed prophylaxis treatment and offered adamant encouragement about better brushing and flossing, and an appointment was made for renewed repair of the tooth.
New Treatment
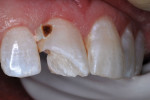
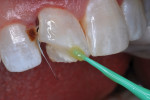
The patient was anesthetized with local infiltration of articaine hydrogen chloride (HCl) 4%, with 1/200,000 epinephrine. The prior RBC material was cut away with a water-cooled high-speed diamond bur. No rubber dam was used. The mouth and throat were protected with a 4”x4” cotton gauze. Carious tooth structure in the distal caries lesion was debrided with slow-speed round burs. Peripheral enamel was roughened with a slow-speed diamond bur to enhance acid-etching, and preparation for RBC repair was completed (Figure 7 and Figure 8).
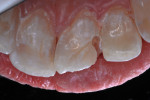
A dentin replacement liner (ACTIVA™ BioACTIVE-BASE/LINER™, Pulpdent, www.pulpdent.com) was used to cover exposed dentin,4 and a metal matrix strip was inserted and stabilized with a wooden wedge. A self-etching resin adhesive (Adper™ Prompt™ L-Pop™ Self-Etch Adhesive, 3M, www.3m.com) was painted on the peripheral enamel and within the preparation, and agitated for 30 seconds (Figure 9). The bonding agent was photopolymerized with 10 seconds of light-beam exposure. The disto-incisal corner of the incisor was then restored in a typical manner with three layers of shade A2B RBC (Filtek™ Supreme Ultra Universal Restorative, 3M); each layer was cured separately with 10 seconds of 1,200 mW cm2 light exposure (Figure 10). A 20-second light exposure from both the labial and then lingual aspects assured thorough resin polymerization. Finishing and polishing was completed in a typical manner.
Two months after re-repair of the tooth, when the tooth was air-dried the patient displayed white enamel dysmineralization of the adjacent surface of the tooth (Figure 11). Enamel microabrasion (PREMA®, Premier Dental, www.premusa.com) was used to remove the white discoloration and modify the enamel surface (Figure 12). Enamelon® Preventive Treatment Gel (Premier Dental) was applied and left in place for 5 minutes after microabrasion was completed (Figure 13), with the intention being that the stannous fluoride and amorphous calcium phosphate in the gel would have a beneficial effect on remineralization of the exposed enamel.
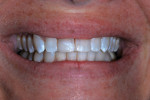
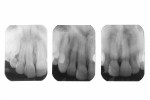
Nine months after re-restoration of the tooth (7 months after enamel microabrasion), the tooth had an improved appearance, whether air-dried or wet with saliva (Figure 14). A comparison of radiographs from 1983, 2015, and 2016 showed no pathological alterations of the root, periodontal ligament space, or associated alveolar bone (Figure 15).
Discussion
At the time of the injury in 1983, white sealant application to the surface of the RBC to simulate the patient’s idiopathic generalized white enamel dysmineralization was considered the optimal treatment option to improve the appearance of the restored tooth. Enamel microabrasion, which might have been a reasonable alternative in combination with RBC repair, was developed after 19832 so it was not considered when the initial tooth repair was rendered. However, 32 years later, enamel microabrasion did prove useful in this case. Some white discoloration adjacent to the RBC (Filtek Supreme Ultra) repair was apparent, even when the tooth was slightly hydrated (Figure 11). A brief application of enamel microabrasion compound (PREMA) not only modified the residual white dysmineralization, but also altered the surface of the enamel such that light was refracted and reflected differently, improving surface appearance (Figure 12 through Figure 14).5
Evolution of RBC Restoratives
When one considers the harsh intraoral environment, including cold and hot thermal insult, constant acidic challenge from the diet, bacteriological influences, and mechanical wear and tear from occlusion and mastication, it is remarkable that a resin-based composite tooth repair can last structurally for more than three decades. Heliosit, the RBC restoration presented in this rare case report, was classified as a conventional microfilled RBC. To better appreciate the significance of this case in the context of more than three decades of evolution of RBC restoratives, it is useful to review the structure of this early class of RBCs.
In a time frame similar to when the microfilled materials appeared, traditional RBCs composed of splintered quartz glass particles were superseded by small-particle RBCs.6 Conventional RBCs, the first generative of these materials, contain relatively large filler (macrofiller) particles such as lithium aluminum glass, ground quartz, or borosilicate glass.7 While most particles range in size from 20 µm to 50 µm, the total range is 0.1 µm to 150 µm. Small-particle RBCs reduced the mean particle size to approximately 5 µm to 10 µm and used borosilicate glass to improve esthetics. Microfilled materials have “microfine” filler particles of pyrolytic silica (silicon dioxide [SiO2]), in the range of 0.007 µm to 0.14 µm with a mean of 0.04 µm.7 However, the large surface area of these very small pyrolytic silica particles limits the amount that can be added directly into the monomer resin composition.
Therefore, to increase filler loading without resorting to the use of larger particle size inorganic fillers, a novel method to create an “organic” filler was used.6 These filler particles in a microfilled RBC consist of pulverized “composite filler particles” dispersed in a pre-cured resin matrix. Pyrogenic colloidal silica particles (0.04 µm) are incorporated into both the pre-cured resin filler particles and the curable monomers; however, the pre-cured resin “organic” particles contain a substantially higher concentration of pyrogenic colloidal silica. As a result, surface roughening and low translucency problems that are typically associated with traditional and small-particle RBCs were solved by using colloidal silica particles as the inorganic filler. Individual particles are approximately 0.04 μm (40 nm) in size, which is 100 to 200 times smaller than the average particle in traditional RBCs and one-tenth of the wavelength of visible light. This optical property of colloidal silica inorganic filler gives microfilled materials an “enamel-like” translucency.8
Furthermore, these materials have a smooth surface, which is similar to the surface texture obtained with the unfilled direct-filling acrylic resins, because the inorganic filler particles are smaller than the abrasive particles used for finishing the restoration. Thus, the silica filler is removed along with the resin in which it is embedded, leaving a very smooth, polished surface that frequently is retained for a significant portion of the life of the restoration.6
Limited Clinical Indications
Nevertheless, this esthetically beneficial microstructure of microfilled RBCs came with some significant limitations. The concept of surface property enhancement with microfilled RBCs entailed reinforcement of the resin primarily by adding organic filler. However, the lack of a strong, chemically stable bond between the organic filler and resin matrix, as well as a relatively high resin-to-filler ratio (only 35 to 50 weight percent pyrolytic silica in both the resin matrix and the prepolymerized organic filler), compromise the microfill’s physical and mechanical properties and limit its clinical indications for use.6,8,9
Through much in vivo experience, it became apparent that the clinical use of microfills should be limited to primarily restoring anterior teeth and cervical areas (such as abfraction lesions). Use of these materials in heavy-stress-bearing restorations is contraindicated because of tendencies for bulk fracture and marginal chipping.6,8 In addition, the microfills also have lower tensile strength, elastic modulus, and fracture toughness, which further compromise their use in higher-stress restorative applications. Other properties of concern are higher thermal expansion coefficients, greater water sorption, and higher levels of polymerization shrinkage, all of which affect marginal adaptation and resistance to long-term surface staining and discoloration.8,10,11
Nanofill Class of Restorative
A high degree of extrinsic staining is clearly evident in the otherwise stable microfilled restoration of 32 years’ duration shown in this report. The quest for optimal strength and esthetics has evolved beyond microfilled RBCs through several generations of RBC classes and compositions. The appearance of “nanohybrid” and “nanofilled” RBCs represents the most recent technological advances to provide both higher strength combined with ideal esthetic properties.
After 32 years in the mouth, Heliosit—the microfill—was replaced by Filtek Supreme Ultra, which is one of the latest formulations developed in the nanofill class of restorative materials. This new group of RBCs contains nanosized filler particles that are synthesized by sol-gel and controlled sintering processes,12 and such materials can offer improved esthetics, reduced polymerization contraction, and enhanced mechanical characteristics. Filtek Supreme Ultra is formed with both nanomer and nanocluster filler particles, while nanohybrid RBCs are hybrid resin composites that contain finely ground glass filler and nanofiller in a prepolymerized filler form. Thus, the filler system in nanofills contains predominantly nanometer-sized particles throughout the matrix; nanohybrids contain more conventional filler technology combined with nanometer-sized particles. In addition to excellent mechanical and physical properties equivalent to other high-strength RBC types, nanofilled RBCs have demonstrated the ability to maintain a high degree of surface continuity and luster. This finding was demonstrated in a 3-year clinical evaluation of Filtek Supreme in anterior teeth documenting performance of this nanofilled resin-based composite in the critical esthetic zone.13
Careful Treatment Protocol
A significant factor in the survival of this 32-year-old tooth restoration was the fact that it was adhesively bonded to properly etched enamel in a well-prepared tooth, using only an unfilled enamel-bonding agent. Although only one tooth is documented here, this report exemplifies the remarkable stability of a micro-mechanical adhesive resin/enamel bond without the need for a dentin-bonding agent. Such careful treatment protocol can obviously have a positive effect on long-term retention and longevity of a Class IV RBC tooth repair.
The dentin replacement liner that was used was applied in a thin layer to replace dentin. Glass-ionomer cement systems chemically bond to dentin with an attachment that does not hydrolyze over time. Such materials make for excellent biocompatible dentin replacement materials and are ideal in the “stratification” restorative method.14
Conclusion
Even with the above outlined improvements in properties of RBCs since 1983, predicting how many years of good service the renewed repair will provide is difficult. The authors are hopeful to have an opportunity to re-evaluate the patient in the far future and perhaps present yet another case report at that time.
Disclosures
Theodore P. Croll, DDS and Steven R. Jefferies, MS, DDS, PhD, have no relevant financial relationships to disclose.
References
1. Croll TP. Emergency repair followed by complete-coronal restoration of a fractured mandibular incisor. Quintessence Int. 1992;23(12):817-822.
2. Croll TP. Enamel microabrasion for removal of superficial dysmineralization and decalcification defects. J Am Dent Assoc. 1990;120(4):411-415.
3. Croll TP. Elimination and simulation of white enamel discoloration. Part II. Quintessence Int. 1984;15 (3):321-328.
4. Croll TP, Berg JH, Donly KJ. Dental repair material: a resin-modified glass-ionomer bioactive ionic resin-based composite. Compend Contin Educ Dent. 2015;36 (1):60-65.
5. Donly KJ, O’Neill M, Croll TP. Enamel microabrasion: a microscopic evaluation of the “abrosion effect.” Quintessence Int. 1992;23 (3):175-179.
6. Anusavice KJ. Phillips’ Science of Dental Materials. 11th ed. Philadelphia, PA: Elsevier; 2003: 421-425.
7. van Dijken JWV. Conventional, Microfilled and Hybrid Composite Resins: Laboratory and Clinical Evaluations. DiVA Portal website. https://www.diva-portal.org/smash/get/diva2:793374/FULLTEXT01.pdf. Accessed May 27, 2016.
8. Milnar FJ. The evolution of direct composites. Compend Contin Educ Dent. 2011;32(1):79-81.
9. Lambrechts P, Vanherle G. Structural evidences of the microfilled composites. J Biomed Mater Res. 1983;17(2):249-260.
10. Luce MS, Campbell CE. Stain potential of four microfilled composites. J Prosthet Dent. 1988;60(2): 151-154.
11. Shintani H, Satou N, Yukihiro A, et al. Water sorption, solubility and staining properties of microfilled resins polished by various methods. Dent Mater J. 1985;4(1):54-62.
12. Croll TP, Jefferies SR. Repair of lemon juice-eroded incisors in a teenager. Inside Dentistry. 2013;9(8):70-77.
13. Dunn J, Munoz C, Wilson A, et al. Three year clinical evaluation of Filtek Supreme in anterior teeth [abstract]. J Dent Res. 2006;85(spec iss A). Abstract 0359.
14. Ruiz JL, Mitra S. Using cavity liners with direct posterior composite restorations. Compend Contin Educ Dent. 2006;27(6):347-351.
About the Authors
Theodore P. Croll, DDS
Affiliate Professor
Department of Pediatric Dentistry
University of Washington School of Dentistry
Seattle, Washington
Adjunct Professor
Department of Pediatric Dentistry
University of Texas Health Science Center at San Antonio
San Antonio, Texas
Private Practice
Doylestown, Pennsylvania
Steven R. Jefferies, MS, DDS, PhD
Professor
Associate Dean for Research & Graduate Education
Department of Restorative Dentistry
Director of the Biomaterials
Research Laboratory
Maurice H. Kornberg School of Dentistry at Temple University
Philadelphia, Pennsylvania