What’s in a Term?
The importance of staying up-to-date on current dental terminology
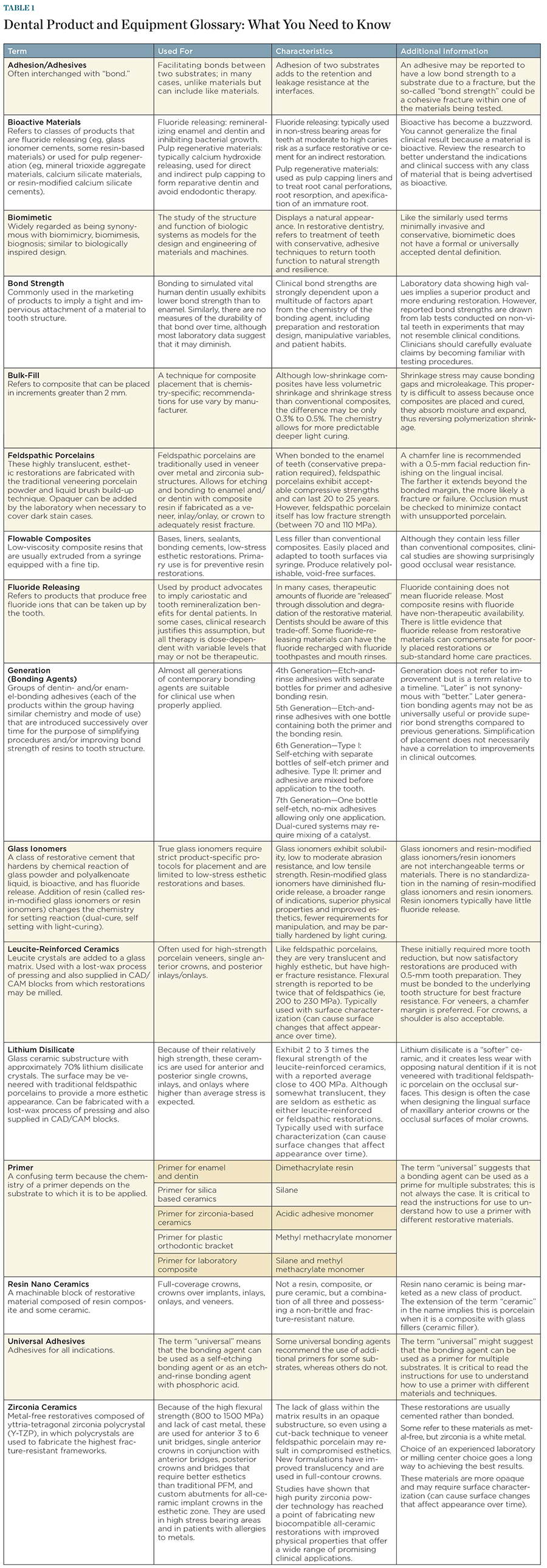
Many clinicians and researchers have outlined the differences between ceramic materials, composites, and types of restorations, along with their respective indications, features, and benefits. The vast array of dental products and materials—and the terminology used to categorize and describe them—create confusion for clinicians. Dental practitioners are left to assess and determine a variety of factors, such as which materials to use for what and when, under what conditions, etc. Whether they are “real” scientific terms or created by marketers, the qualifying descriptors being used to differentiate newly introduced products from a previous formulation or version or those terms created to describe something entirely new can be confusing, misleading, and/or incorrect (Figure 1 through Figure 4; download a full version of the table for your reference here).
The Current State of Dental Marketing
“What’s happening in dental marketing is the creation of new names and classifications that wouldn’t have a definition or descriptor if one were to consult traditional biomaterials textbooks, articles, and organizations,” says Howard Strassler, DMD, professor and director of operative dentistry in the department of general dentistry at the University of Maryland School of Dentistry. “Many descriptors are part of product naming that includes plus, ultimate, ultra, and max; and universal and all-in-one, just to name a few.”
There also are such descriptors as nano, which seems to be tied to many products, and hybrid, a catch-all phrase for a combination of materials, Strassler observes.
“Marketers often distort or use terms that can have multiple meanings, and they don’t define their terms,” notes Edward F. Rossomando, DDS, PhD, MS, professor in the division of craniofacial sciences at the University of Connecticut School of Dental Medicine. “In everyday life, most people are familiar with marketing phraseology, with terms such as ‘new and improved’ or ‘fast-acting,’ but these terms are deliberately used because they are ambiguous and have no specific meaning.”
Astute consumers will question—new and improved over what? The one before? If so, by how much? How fast is fast-acting, and compared to what?
The typical half-life of a dental product is 18 months, says John M. Powers, PhD, senior vice president and senior editor of The Dental Advisor. Companies remain competitive by introducing improved products and then changing the product name. In doing so, they often maintain their brand name but add terms (eg, Plus, Improved, II) to indicate that the product is new or improved. These improvements might include changes in packaging to make manipulation easier, or in formulation to improve clinical performance.
In today’s dental product and equipment world, many of the improvements made to restorative materials are not based upon clinical trials, but on bench testing, Strassler says. Years ago, improvements were significant, but today they are not as statistically substantial; yet, those improvements allow manufacturers to compare their products more positively to their competition.
“Manufacturers change chemistries and ‘tweak’ products, enabling them to show some improvements,” Strassler elaborates. “They’re trying to make them easier to use, but are these changes clinically substantial, and will they improve the quality of care we provide our patients?”
Powers observes that dental terminology is continually evolving as new formulations and novel packaging expand the uses of dental products, perhaps leading to improved clinical performance.
As a result, readers of dental publications may encounter the intentional “creative” use of ordinary terms in advertisements that imply clinical benefits that are not supported by evidence, observes Alton Lacy, PhD, DDS, professor emeritus at the University of California, San Francisco School of Dentistry, in the department of restorative and preventive dental sciences.
“Thoughtful peer-review will catch most of these errors before articles are published, but non-reviewed articles often require an extra measure of scrutiny by the dental reader,” Lacy says.
Click on the Table below for a full-size, printable version:
Dental manufacturers typically use current terms or develop new terms to indicate the improved handling, properties, or expanded use of their products, Powers explains. Sometimes, terms are based on chemistry, but terms are often creations of marketing people.
“What these terms mean to dental practitioners has been a challenge,” says Rossomando. “It’s not until they use a product chairside and see how it actually works that dental practitioners can superimpose their own meaning to the descriptors that marketers use.”
For example, when addressing bond strength, composite shrinkage, and/or slight changes in chemistry, some dentists may neglect the fact that curing lights may not be well calibrated, Strassler says. Therefore, it can be questionable as to whether or not they deliver sufficient energy and completely polymerize composites. A clinical problem with a restorative material may not be in its chemistry, but related to how the clinician places it.
“Composites require a certain amount of energy in order to harden, and the curing lights used may not adequately deliver the energy over the recommended time,” Strassler points out.
Additionally, it’s not unusual to find unintentional misuse of terms in dental literature that either make no sense or are scientifically inaccurate, notes Lacy. In these cases, the reader may be confused, and/or the author’s credibility is challenged. For example, he recalls an article he read in which the authors referred to zirconia (ie, porcelain) as zirconium (ie, a metallic element). In another instance, silicon dioxide, which is properly known as silica, was referred to as silicium (no such word) oxide.
“Inappropriate use of scientific terms may lead one to question an author’s depth of knowledge of a subject,” Lacy cautions.
Standard Terminology
Powers notes that a lack of standardization causes confusion. Concerning as it may be, it is reasonable to ask, are dental practitioners aware of standard terminology and do manufacturers actually use standard terminology?
Over many years, dentistry has recognized the need for standard definitions of terminology, Powers says. Some examples of attempts to provide standard dental terms and definitions have included the FDI Lexicon of English Dental Terms (Baume LJ, International Dental Federation), Boucher’s Clinical Dental Terminology (Zwemer TJ, Mosby), ANSI/ADA Specification No. 33, and ISO 1942.
The terms that are most current today for ceramic materials, for example, are based upon the fabrication process and/or composition of the restoration provided, explains John Calamia, professor and director of esthetics at New York University College of Dentistry. Modern dental laboratories offer an assortment of restorations, and it is up to the dentist/technician partnership to identify what will work best for the patient considering the underlying tooth structure remaining and its surrounding tissues.
“Also coming into play will be the physics or mechanics of the forces that will be involved, now and in the future, and the room necessary to provide the best esthetic result that can be attained while delivering the best long-term result,” Calamia says. Understanding the occlusion and any parafunctional concerns must be identified and brought into the material selection process. Main categories might include feldspathic porcelains, lithium disilicate products, zirconium products, and whether they are built up freehand, milled, or milled and layered with external freehand additions.
The best way for dentists to eliminate any gray area created by the terminology used to characterize porcelain and all-ceramic products is to remember that each case should be judged on its individual needs and problems, Calamia advises. The clinician must have a final vision of the type of restorations necessary for the treatment. Then he or she must work backwards to determine what type of material(s) will be used for these restorations.
“We try to consider the most conservative preparation or loss of tooth structure without abandoning the patient’s desire for a totally esthetic result,” Calamia says. “Considerations must include the teeth to be prepared, the surrounding tooth structure, and the occlusion (ie, stresses that currently exist or will exist at the end of treatment).” Often, the stronger the ceramic material used, the less esthetic the result. Combinations of different ceramic materials might not provide the long-term esthetics one looks to in a valuation of the success of the case.
The clinician may need a full wax-up of the proposed treatment to help ascertain, with assistance from laboratory technicians, what specific material or combinations of materials should be used, Calamia suggests. Using specific terminology for available ceramic materials and understanding the capabilities of the different categories should make that communication easier, he adds. Strassler says that for some clinical procedures, the traditional diagnostic wax-up is necessary, but the addition of diagnostic digital imaging mock-up/“wax-up” can portray expected results that a traditional wax-up may limit.
Clearing Through the Chaos
“Dentists have to understand why they’re making a change from one product to another. Are they changing products because what they previously used are no longer manufactured, for the sake of change, or to realize a true benefit?” Strassler emphasizes. “Dentists should be making changes to the products they use because they’re unhappy with the clinical results with what they’re using now.”
Rossomando advocates word-of-mouth recommendations, observations from peers, and insight garnered from study clubs as a means to evaluate and define descriptive product terminology. He also suggests reviewing the findings from organizations that conduct independent evaluations and product testing.
“Product evaluation services, such as The Dental Advisor and Clinicians Report, provide independent and objective testing of products with claims of improved properties,” Powers notes. “The Dental Advisor provides results from evidence-based laboratory and clinical evaluations of new and improved products and tracks patients with restorations to determine long-term clinical performance.”
Additionally, to reduce confusion, dental practitioners should read directions for use (DFU) and refer to the Material Safety Data Sheets (MSDS) provided with dental products, Powers suggests. They can remain current with evolving terminology by having in-office lunch-and-learn sessions with company representatives, attending continuing education programs, and reading newsletters and magazines that describe and evaluate product improvements.