The Failing Implant: Reducing Risk
Addressing modifiable risk factors to help avoid complications
In 2006, the number of implants placed in the United States was estimated at 500,000 a year, with 10% of all dentists placing implants.1 The growth rate for implants has been estimated at 12% each year, but that may have been conservative.2 It is a billion dollar business and growing, with general dentists placing more than 3 million implants compared with about 2 million for dental specialists.1
Dental implants are often the first choice for replacing missing teeth, especially for fully edentulous patients. The success rate of dental implants has improved significantly over the years, and is often touted at 90% to 95%.3,4 Those with minimal surgical experience tend to have more complications,4,5 which may be due to a lack of treatment planning or training.
Patients expect implants to be successful, but they may not understand the important role they play in the treatment process. To promote implant longevity, there are a few steps practitioners can take that will help patients understand the benefits, risks, and importance of adherence to a maintenance program. Even with proper patient education, in some cases there may still be complications that have to be addressed.
Reducing Implant Complications and Failures
It is logical to think changes to modifiable risk factors associated with implant complication and failure will improve long-term outcomes. This is easy in theory, but in reality, behavior change is not easy. Several biologic and behavioral risk factors may affect implant success, including smoking, diabetes, biofilm, and history of periodontal disease. Nutrition and stress are just as important and should be assessed with every implant patient.
Nutrition
It is important to assess a patient’s nutritional status before surgery. Studies have shown that improving nutritional status will provide the necessary building blocks for optimal wound healing.6 One of the most studied is the effect of vitamin C deficiency on wound healing. Assessment of vitamin A, D, E, and mineral intake (such as calcium, magnesium, and boron), is also recommended.7
Stress
Stress can affect implant survival in different ways. Mental stress has been shown to decrease wound healing, and stress reduction techniques have been shown to shorten healing time.8 Stress can also manifest as clenching or bruxism, which can affect long-term implant survival. This can result in implant overload and eventually bone loss or even implant fracture.
Smoking
The literature shows that smoking is a major risk factor for periodontal disease. Implant patients who smoke are at increased risk for peri-implantitis, and have increased probing pocket depths, bleeding on probing, marginal bone loss, and loss of implants compared with non-smoking implant patients.9
Diabetes
The association between diabetes and periodontal disease is well researched, showing a bi-directional relationship, more severe disease, and less favorable prognosis after treatment, especially in those with poor glucose control, undiagnosed disease, or poor oral hygiene. Studies specific to the impact of diabetes and implants are minimal, but patients with poor glycemic control have been reported to have an increased risk of peri-implantitis and elevated pro-inflammatory mediators at the implant sites.10
Biofilm
The initiation of peri-implant disease is bacteria or overload, or both. However, the extent and severity of the infection is related to the host response and inflammatory process. The microflora around implants with peri-implantitis is not that different from periodontal disease. It is dominated by gram-negative bacteria, with higher numbers of pathogenic organisms in the subgingival flora.11
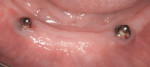
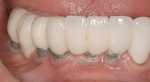
Mucositis is inflammation of the mucosal tissue, similar to gingivitis around teeth, and is reported in 80% of patients (Figure 1). Inflammation with bone loss is peri-implantitis and occurs between 28% and 56% of patients (Figure 2).12 Assuming proper implant and osseointegration, these results imply there are complications associated with biofilm control either caused by the placement of non-cleansable prosthesis, foreign body such as retained cement, or poor oral hygiene.
History of Periodontal Disease
The history of periodontal disease is not a contraindication for implant placement, but patients should be informed that they are at an increased risk for peri-implant disease. Patients may assume an implant is resistant to infection when it replaces an extracted periodontally compromised tooth. Studies show they are just as susceptible due to the host response to biofilm and other potential risk factors. Addressing the patient’s risks, eliminating the infection, and adherence to an oral hygiene regimen can improve the longevity of the implant for these patients.13
Best Practices
Dental professionals strive to provide the best treatment and prevent errors and complications, especially during surgical procedures. Errors vary greatly among dental professionals and are associated with inexperience, non-adherence to accepted practice protocols, patient selection and preparation, emergency procedures, and collaboration with other healthcare providers. Studies have shown a reduction in complications and errors with the use of checklists to reduce morbidity and mortality in hospitals and other settings. Recently, Christman and colleagues conducted a Delphi study to design a checklist for implant placement. The researchers chose 24 board-certified periodontists as panelists; each had been placing implants for 5 or more years, placed a minimum of 1,000 implants, and focused primarily on implants in the practice.14 The resulting checklist has three areas: treatment planning phase, intraoperative phase, and postoperative phase. It provides a systematic approach to implant treatment based on expert opinion and experience, but has not been field tested to determine if is clinically useful and will reduce errors. A maintenance checklist for continued care of the implant and patient is also needed.
Treating Peri-Implant Disease
Peri-implant diseases have been treated non-surgically in the early stages, and surgically if pocket depth is greater than 7 mm.15 It is important to note there is no standard of care to date related to a protocol to treat peri-implant diseases.
Nonsurgical Treatment
Most nonsurgical therapy decisions for peri-implant disease are based on the evidence for periodontitis. The goal is to eliminate the infection, remove the biofilm from the implant surface, and provide effective ways to clean the implant (Table 1 and Table 2). Removal of the biofilm around the implant and prosthesis with ultrasonic instruments using a non-metal tip is best, to avoid damaging the implant surface.15
The use of local antibiotic therapy in conjunction with local debridement around implants has been evaluated in case reports and shown to be effective in mild to moderate peri-implantitis. A few studies compared different treatment modalities with modest differences in clinical parameters. Minocycline HCL microspheres, doxycycline hyclate, and tetracycline fibers have all been shown to be effective in minimizing inflammation and decreasing pocket depths around the implants.16-18
Systemic antibiotics may be useful to treat peri-implantitis, depending on the severity of the infection, but more studies are needed to establish a protocol.19 Identifying bacteria present at the site through culture or DNA testing may be useful in determining course of treatment and type of antibiotic to be used.15 Generally, if suppuration, diffuse redness, swelling, and pain are noted, an antibiotic should be prescribed following the protocol for a tooth abscess.
Different types of lasers have been used as adjuncts to treatment of peri-implantitis; however, more studies are needed to determine if they are superior in reducing the pocket depth around implants compared with local debridement alone.20 The Er:YAG laser has been used to treat peri-implantitis but did not show significant benefit over scaling followed by chlorhexidine irrigation and gel placement or over the use of subgingival air abrasion.21 Using the CO2 laser to debride the contaminated implant surface showed promising results, but more studies are necessary to confirm its efficacy.22
Patient nonadherence to an oral hygiene protocol may be the primary reason for infection; this can stem from difficulty complying with the recommendations, lack of understanding of their importance, or inadequate access due to an oversized prosthesis. These issues need to be addressed and modifications made. If the area is not cleanable by the patient, changes to the prosthesis may be necessary, along with shorter office maintenance intervals.
Surgical Treatment
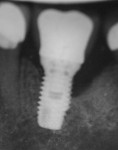
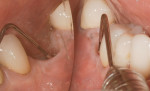
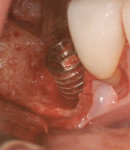
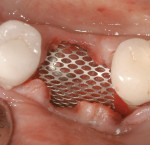
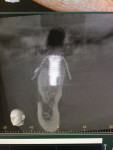
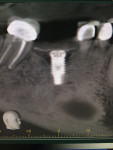
Surgical intervention in treating peri-implantitis is indicated if pocket depth is greater than 7 mm.15 This consists of opening a full thickness flap, debriding the implant surface, and bone regeneration in conjunction with resorbable or non-resorbable membranes (Figure 3 through Figure 10). When treating circumferential bone loss, the crown and abutment are removed so the treated implant can be taken out of function by submerging the implant fully under the soft tissue during the healing period (4 to 6 months). If more than 50% of the bone has been lost around the implant, or if mobility is present, implant removal is indicated.15
A review of studies on surgical treatment of peri-implantitis shows many positive short-term results. The results of surgical treatment are inconsistent and treatments vary among practitioners, however, making a good meta-analysis difficult to perform. In many studies, a lack of disease resolution or a return of peri-implantitis exists.23 One of the reasons for suboptimal outcomes may be an inability to completely detoxify and remove the bacteria from the implant surface. Because of this inconsistency in the peri-implantitis treatment studies, no protocol has been established as the standard of care.
It is important to recognize and address causes of peri-implantitis, as well as minimize risk factors. Assessment of the thickness and width of keratinized gingiva around implants is an important step in preventing future bone loss or peri-implantitis. Thin, loose gingiva can harbor food and plaque, and increase inflammation around an implant. Augmentation to increase width and thickness of soft tissue around implants at the gingival margin may be necessary, and is best performed in the early stages of peri-implant disease, or even better, at the time of implant placement if thin gingiva is present.
Maintenance Protocol
One of the most important components of implant longevity is patient self-care. There are many products on the market that can be recommended, but few have been studied with implants, especially those that limit access, such as full-arch fixed prosthesis and overdenture bars. Mouth rinses may be helpful if they can access the gingival margin, floss is useful if it is easily threaded around the prosthesis, and power toothbrushes are safe to use and have been shown to be effective in limited studies. A recent study compared use of an oral irrigator with string floss for single implant replacement, and demonstrated a significantly better reduction in bleeding over 30 days.24 Daily irrigation may be one of the best tools, as the pulsating water or other solution can access areas that other devices cannot reach. It also has the ability to clean deeper pockets that may be normal around the implant.
Continued care is the final stage of implant placement. To date, there is no standard of care for maintaining implants. This is not unexpected, as there are many areas that require research, but for now, clinical decision and expertise, and perhaps a little creativity, drive recommendations. Patients should be placed on a personalized support program based on their needs and risk factors. The goal is to prevent infection or relapse in patients who have previously been treated for peri-implant disease.
Conclusion
Dental implants are the best replacements for missing teeth, and have one of the highest success rates of any medical and dental surgery. It is important to identify risk factors of implant failures before implant placement to ensure our patients have the best possible experience with their dental implant rehabilitation. Regular and thorough assessment of the patient’s overall health and well-being is a must. We cannot fully escape the biofilm, but as a team, we can control the amount of bacteria through good oral hygiene practices, modification of host response by decreasing inflammation via medical and nutritional interventions, and removal of excessive forces that may cause bone loss or implant fractures.
Disclosure:
Dr. Moldovan is a consultant for Water Pik, Inc.
References
1. Dental Implants Facts and Figures. American Academy of Implant Dentistry website. www.aaid.com/about/Press_Room/Dental_Implants_FAQ.html. Accessed April 14, 2014.
2. Millennium Research Group. U.S. markets for dental implants 2001: executive summary. Implant Dent. 2001;10(4):234-237.
3. Nixon KC, Chen ST, Ivanovski S. A retrospective analysis of 1,000 consecutively placed implants in private practice. Aust Dent J. 2009;54(2):123-129. doi: 10.1111/j.1834-7819.2009.01104.x.
4. Pjetursson BE, Brägger U, Lang NP, Zwahlen M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin Oral Implants Res. 2007;18(suppl 3):97-113. Erratum in Clin Oral Implants Res. 2008;19(3):326-328.
5. Kourtis SG, Sotiriadou S, Voliotis S, Challas A. Private practice results of dental implants. Part I: survival and evaluation of risk factors—Part II: surgical and prosthetic complications. Implant Dent. 2004;13(4):373-385.
6. Dryden SV, Shoemaker WG, Kim JH. Wound management and nutrition for optimal wound healing. Atlas Oral Maxillofac Surg Clin North Am. 2013;21(1):37-47. doi: 10.1016/j.cxom.2012.12.008.
7. Carr AC, Vissers MC. Good nutrition matters: hypovitaminosis C associated with depressed mood and poor wound healing. N Z Med J. 2012;125(1362):107-109.
8. Broadbent E, Kahokehr A, Booth RJ, et al. A brief relaxation intervention reduces stress and improves surgical wound healing response: a randomised trial. Brain Behav Immun. 2012;26(2):212-217. doi: 10.1016/j.bbi.2011.06.014.
9. Strietzel FP, Reichart PA, Kale A, et al. Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis. J Clin Periodontol. 2007;34(6):523-544.
10. Rinke S, Ohl S, Ziebolz D, et al. Prevalence of periimplant disease in partially edentulous patients: a practice-based cross-sectional study. Clin Oral Implants Res. 2011;22(8):826-833. doi: 10.1111/j.1600-
0501.2010.02061.x.
11. Mombelli A, Décaillet F. The characteristics of biofilms in peri-implant disease. J Clin Periodontol. 2011;38 Suppl 11:203-213. doi: 10.1111/j.1600-051X.
2010.01666.x.
12. Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):282-285. doi: 10.1111/j.1600-051X.2008.01283.x.
13. Renvert S, Persson GR. Periodontitis as a potential risk factor for peri-implantitis. J Clin Periodontol. 2009;36 Suppl 10:9-14. doi: 10.1111/j.1600-051X.2009.
01416.x.
14. Christman A, Schrader S, John V, et al. Designing a safety checklist for dental implant placement: a Delphi study. J Am Dent Assoc. 2014;145(2):131-140. doi: 10.14219/jada.2013.15.
15. Misch CE. Contemporary Implant Dentistry, 3rd ed. St. Louis, MO: Mosby; 2008.
16. Mombelli A, Feloutzis A, Brägger U, Lang NP. Treatment of peri-implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. [Article in English, French, German] Clin Oral Implants Res. 2001;12(4):287-294.
17. Büchter A, Meyer U, Kruse-Lösler B, et al. Sustained release of doxycycline for the treatment of peri-implantitis: randomised controlled trial. Br J Oral Maxillofac Surg. 2004;42(5):439-444.
18. Renvert S, Lessem J, Dahlén G, et al. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: a randomized clinical trial. J Periodontol. 2008;79(5):836-844. doi: 10.1902/jop.2008.070347.
19. van Winkelhoff AJ. Antibiotics in the treatment of peri-implantitis. Eur J Oral Implantol. 2012;5 suppl:S43-S50.
20. Kotsakis GA, Konstantinidis I, Karoussis IK, et al. A systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis [Published online ahead of print January 20 2014]. J Periodontol.
21. Renvert S, Lindahl C, Roos Jansaker AM, Persson GR. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol. 2011;38(1):65-73. doi: 10.1111/j.1600-051X.2010.01646.x.
22. Romanos G, Ko HH, Froum S, Tarnow D. The use of CO(2) laser in the treatment of peri-implantitis. Photomed Laser Surg. 2009;27(3):381-386. doi: 10.1089/pho.2008.2280.
23. Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29 suppl:325-345. doi: 10.11607/jomi.2014suppl.g5.3.
24. Magnuson B, Harsono M, Stark PC, et al. Comparison of the effect of two interdental cleaning devices around implants on the reduction of bleeding: a 30-day randomized clinical trial. Compend Contin Educ Dent. 2013;34(spec no 8):2-7.
ABOUT THE AUTHORS
Sanda Moldovan, DDS, MS, CNS
Diplomate, American Academy of Periodontology
Private Practice
Beverly Hills, California
Deborah M. Lyle, RDH, BS, MS
Director of Professional and Clinical Affairs
Water Pik, Inc.
Fort Collins, Colorado