Managing the Clinical Risks to Bonding
Retentive design determines proper adhesive protocols.
By Douglas J. Brown DDS | Garrick Alex | Taylor Brown | Angela Suh
Whose “material truth” is the clinician to believe and at what risk? There has been increased interest from dental professionals for products that offer simplicity or tout multiple substrate effectiveness. Are we as a profession willing to accept product simplicity or a manufacturer’s bias as the motivating factor for a material’s use rather than assess the material’s effectiveness by itself or coupled with synergistic materials when addressing the challenges that could lead to clinical failure? The ability to predictably bond and seal tooth substrates to indirect substrates could allow dentists to predictably manage the clinical risks associated with failures. Providing a hydrophobic seal is facilitated with the use of adhesives, primers, and resin cements, which can be used separately or in combination, dependent upon retention and resistance form.
Leakage is the clinician’s “worst enemy” and the use of adhesives, cements, or indirect primers that are less than compatible could lead to the clinician’s “worst nightmare.” 1 Research states that most crowns are at an immediate risk of silently leaking in part as a result of the inherent difficulty in creating retentive preparation designs2 and the lack of attention paid to the use of proper adhesive protocols on indirect substrates, which contributes to durability of the restoration.3
Revisiting Retention/Resistance Form
Some clinicians and manufacturers may claim that conventional luting or self-adhesive cements/glass ionomers provide adequate adhesion when bonding most indirect restorations to tooth substrates. How would retention/resistance form affect this statement?
Research supports the claim that most preparation designs in clinical practice average > 20º tapers which would dictate the use of adhesive cementation using separate adhesive primers4 (Figure 1 and Table 1). When preparation designs are fully retentive (strong adhesion to tooth substrate is not critical), non-adhesive cements or mildly adhesive cements, such as glass-ionomer cements or self-adhesive resin cements (Bis Cem®, Bisco, Inc., www.bisco.com; RelyX™ Unicem, 3M ESPE, www.3MESPE.com), can be used. Sealing the indirect substrate with primers should be considered. However, based on tooth placement and loss of tooth structure, most typical preparation designs may include less than ideal retentive designs. For restorations with less than optimal retention form, the use of dual-cure resin cement, enamel/dentin bonding agents, and indirect substrate primers should be considered3,5.
Dual-cured cements are preferred over light-cured–only cements when the potential for limited light transmission exists, such as with opaque cores or thick indirect substrates. Dual-cured cements and adhesive systems are not created equal. Most do not perform equally well, especially in the self-cure mode. Self-cure mode rules in indirect dentistry and it is imperative that the clinician has confidence in the curing characteristics of the materials to be used.
Priming the Indirect Substrate
The importance of improving the interaction of cement to the internal surface of the new and improved indirect substrates currently used in dentistry—zirconia (Lava™, 3M ESPE; Cercon®, DENTSPLY International, www.dentsply.com) or metal and lithium disilicate (IPS e.max®, Ivoclar Vivadent, www.ivoclarvivadent.com) —is a hot topic. Clinicians covet adhesive systems that address this issue with predictability. But are all primers the same? How do they differ and what is important for the clinician to know? Recent International Association for Dental Research 2011 abstracts explored these questions using current scientific methods and presented with some interesting findings.6
Bonding to Porcelain/Ceramic
Adhesion is both mechanical and chemical in nature. Bonding of ceramics to tooth structure is well researched and documented, yielding strong, predictable, and durable bonds. A strong immediate bond to ceramic relies on sandblasted/hydrofluoric (HF) acid-induced micromechanical interlocking to the ceramic surface.7-9
The subsequent application of silane coupling agents to previously HF acid-etched porcelain provides for chemical bonding (Figure 2). Silane chemical bonding is shown to correlate with improved durability of long-term bond potential and less with immediate mechanical bond strengths. There are at least three types of silane primers currently on the market, including traditional silane primers and silane primers with additives (ie, extra resin or acidic phosphate monomers) (Table 2).
Are all silane-containing products the same? Will acidic monomer or resin additives render silane-containing products less effective? What effect does aging have on loss of effectiveness?
The efficacy of chemical bonding was shown, by indirect means, using contact angle measurements as well as bond strength testing.6,10 Contact angle is the angle at which water and surface meet and represents degrees of hydrophobicity or hydrophilicity. A hydrophobic surface, represented by a high contact angle, is desirable to optimize interaction with subsequently placed hydrophobic resin-based cements and adhesives and may correspond with higher bond strength numbers.
Results
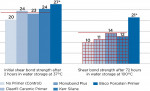
Based on the silane chemistry tested, traditional silane primers containing no extra additives such as Porcelain Primer (Bisco, Inc.) are recommended for silica-based (glass) ceramic restorations, such as lithium disilicate, porcelains, etc. All other silane primers with additives are much less effective for the treatment of ceramics (Figure 3).
Silane primers containing extra acidic phosphate monomers. The acidic phosphate monomers could accelerate the conversion of active pre-hydrolyzed silane. Literature has shown silane to polymerize via condensation reaction to form polysiloxane oligomer under acidic conditions11 (pH < 4) and has resulted in manufacturers stabilizing pre-hydrolyzed silane to a pH much higher than 4. The pH of the phosphate monomer-containing silane primers (Monobond Plus, Ivoclar Vivadent; CLEARFIL™ Ceramic Primer, Kuraray America Co., www.kuraraydental.com) are well below 4. In contrast, the pH of Bisco’s Porcelain Primer is 5.
Silane primers containing extra resin. Firstly, the extra resin could block the reaction site to ceramics, resulting in less covalent bond formation between silane and silica. Secondly, the covalent bond formation is a condensation reaction and the extra resin could retard the evaporation of water molecules.
Bonding to Zirconia and Metal Oxides
Zirconia, a silica-free, acid-resistant, polycrystalline ceramic, does not contain amorphous silica glass, which makes traditional ceramic surface treatments (such as HF etching and/or silane primer application) ineffective. Polycrystalline zirconia requires specific primers, which are used to form chemical bonds. Indirect evidence of chemical bonding is shown through both examination of scanning electron microscope-fractured specimens and contact angle measurement analysis.
Alteration of the internal surface of zirconia with a bur/diamond or sandblasting before cementation may increase surface area and bond strengths, but is controversial. Research shows that zirconia is susceptible to phase transformation by mechanical means from the tetragonal to monoclinic crystalline structures, which induces volume increases and surface stresses.12 Additionally, the use of tribo-chemical treatments (Cojet™/Rocatec™, 3M ESPE) may be prone to degradation, and studies show these treatments do not offer significant improvement on bond strength.13 International Association for Dental Research 2011 abstracts explored the use of primers to metal oxides.14-16
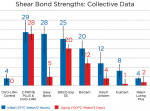
There are four commercially available zirconia primer systems currently on the market: Clearfil Ceramic Primer, AZ Primer (Shofu Dental, www.shofu.com), Monobond Plus, and Z-PRIME™ Plus (Bisco, Inc.). Z-PRIME Plus is the only phosphate monomer formula of this group to provide direct evidence of chemical bond formation with the use of secondary ion mass spectrometry (Table 3).16
Discussion
Primers significantly improved levels of hydrophobicity. The primers/adhesives tested, in conjunction with hydrophobic resin cements, show the highest shear bond strengths. After 3 days of aging in boiling water, Z-PRIME Plus (with hydrophobic cement) showed stable bond durability, with all other systems showing a significant decrease of bond strength (Figure 4).
Conclusion
Restoring the worn dentition via indirect means is clinically challenging. Clinicians have a professional responsibility to create durable restorations. Adhesive protocols and materials should be used as a tool for managing the risks that could potentially lead to failure. It is the role of manufacturers to create products that best serve the dental profession and it is the role of researchers to challenge thought processes and motivate the next generation of product development.
Authors’ Note
The research data was generated during a summer 2010 internship and culminated in both oral and poster presentations during the 2011 International Association for Dental Research meeting in San Diego, California.
References
1. Nordlander J, Weir D, Stoffer W, et al. Taper of clinical preparations for fixed prosthodontics. J Prosthet Dent. 1988;60(2):148-151.
2. Sorensen JA, Kang SK, Avera SP. Porcelain-composite interface microleakage with various porcelain surface treatments. Dent Mater. 1991;7(2):118-123.
3. Zidan O, Ferguson GC. The retention of complete crowns prepared with three different tapers and luted with four different cements. J Prosthet Dent. 2003;89(6):565-571.
4. Goodacre CJ, Campagni WV, Aguilino SA. Tooth preparations for complete crowns: An art form based on scientific principle. J Prosthet Dent. 2001;85(4):363-376.
5. Chen JH, Matsumura H, Atsuta M. Effect of etchant, etching period, and silane priming on bond strength to porcelain of composite resin. Oper Dent. 1998;23(5):250-257.
6. Chen L, Alex G, Brown T, et al. Shear bond strengths of different types of silane primers. Poster presented at: International Association for Dental Research Meeting; March 17, 2011; San Diego, CA.
7. Ferrando JM, Graser GN, Tallents RH, Jarvis RH. Tensile strength and microleakage of porcelain repair materials. J Prosthet Dent. 1983;50(1):44-50.
8. Semmelman JO, Kulp PR. Silane bonding porcelain teeth to acrylic. J Am Dent Assoc. 1968;76(1):69-73.
9. Wolf DM, Powers JM, O’Keefe KL. Bond strength of composite to porcelain treated with new porcelain repair agents. Dent Mater. 1992;8(3):158-161.
10. Chen L, Alex G, Brown T, et al. Priming efficacy of three types of silane primers. Presented at: International Association for Dental Research Meeting. March 17, 2011; San Diego, CA.
11. Antonucci JM, Dickens SH, et al. Chemistry of Silanes: Interfaces in Dental Polymers and Composites. Journal of Research National Institute of Standards and Technology. September/ October 2005;110:541-558.
12. Sato H, Yamada K, Pezzotti G, et al. Mechanical properties of dental zirconia ceramics changed with sandblasting and heat treatment. Dent Mater J. 2008;27(3):408-414.
13. Chen L, Suh BI, Kim J, Tay FR. Evaluation of silica-coating techniques for zirconia bonding. Am J Dent. 2011; 24(2):79-84.
14. Chen L, Alex G, Brown T, et al. Metal-bonding: contact angles on primed metals and bond strength tests. Presented at: International Association for Dental Research Meeting; March 16, 2011; San Diego, CA. Oral Session 133.
15. Chen, L, Brown D, Suh BI. Zirconia-bonding 1: bond strength of different bonding systems to zirconia ceramics. Presented at: International Association for Dental Research Meeting. March 17, 2011; San Diego, CA.
16. Chen, L, Brown D, Suh BI. Zirconia-bonding 1: Chemical bond formation between ZPRIME and Zirconia Ceramics. Presented at: International Association for Dental Research Meeting. March 17, 2011; San Diego, CA.
About the Authors
Douglas J. Brown, DDS
Senior Manager of Clinical Affairs
Bisco, Inc.
Shaumburg, Illinois
Garrick Alex
Third-Year Student
College of Dental Medicine
Columbia University
New York, New York
Taylor Brown
Third-Year Student
University of Michigan
Ann Arbor, Michigan
Angela Suh
Third-Year Dental Track Student
University of Illinois
Chicago, Illinois