Atraumatic Extraction: Advantages and Implementation
Robert A. Horowitz, DDS; Ziv Mazor, DMD
There are numerous advantages of atraumatic extraction and ways to perform this procedure with more predictability.
After 45 years of documented use of endosseous, root-form dental implants, the scope and types of treatment rendered has gone through many phases of “evolution.” In the early days of implant dentistry, the majority of documented cases were either full-arch or in the anterior mandible.1,2 As implant and prosthetic designs improved, the number of segmental and single-tooth cases and placement of implants in grafted sites has dramatically increased.3 The shift has gone from success rates in the 80% range in more dense bone and lower in the posterior maxilla and posterior mandible,4 to approaching better than 95% in all portions of the mouth.5 To maintain these success rates, implants must be placed in ideal prosthetic locations and where there is sufficient quality and quantity of bone. Patients expect implant survival and look for life-like implant-supported restorations. Achieving these goals necessitates preservation and/or augmentation of the gingival tissues at the same surgical procedure, if possible. This article will demonstrate the advantages of atraumatic extraction and ways to perform this procedure with more predictability.
Clinical Scenarios
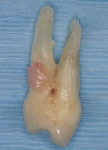
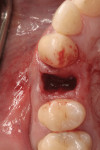
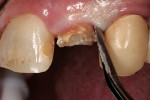
After extensive carious breakdown, tooth fracture, severe periodontal bone loss, and other situations, teeth require extraction (Figure 1). When a tooth is deemed “non-restorable,” the patient and dentist agree that predictable, long-term preservation of the existing tooth will cost more in terms of money, time, and possible lost gingival and supporting bone than extracting the tooth. Minimizing the trauma to both hard and soft tissues simplifies the extraction procedure for the patient. A number of instruments and technologies are currently on the market to assist the surgeon in implementing atraumatic extraction therapy. These range from simple hand instruments to powered and ultrasonic, piezoelectric devices. The ultimate goal of all of these tools is to enable extraction of all of the tooth roots without removing or fracturing the surrounding, supporting alveolar bone.
Ideal analysis of the tooth and local anatomy can only be accomplished through 3-dimensional radiography (Figure 2). While not required for all extractions, the additional information is critical to performing true atraumatic surgery. Knowledge of the number and height of remaining alveolar walls after the tooth is extracted is critical in the surgeon’s choice of regenerative materials and surgical techniques employed in the reconstruction process. While tooth roots may have a normal-appearing anatomy on conventional radiographs, severe dilacerations may be evident when viewing these teeth from other angles. Reconstruction images from cone-beam tomography facilitate patient education, as well as information transfer between the surgeon and other parts of the restorative team who will work on the patient to restore him or her to ideal form and function.
Before beginning any extraction, the tooth shape, root anatomy, and local anatomy must be well identified. If there are potential anatomic complications, the surgeon must be aware of these before beginning the surgical procedure (Figure 3). Leaving a fractured root tip may necessitate full-thickness flap elevation before removal. Similarly, perforating the maxillary sinus is a treatable complication. Depending on the surgical expertise of the operator and location of the problem, repair may or may not be accomplished without adding a significant amount of time and difficulty to the surgery. Other complications are much more challenging to deal with. If a root tip is pushed into the maxillary sinus, a much more extensive surgical procedure will be required to retrieve it. Compression or trans-section of the inferior alveolar nerve has permanent morbidity for the patient. Flap elevation or anatomic variation may lead to the exposure of an arteriole with concomitant significant bleeding. Fracture of the buccal and/or lingual plate will greatly decrease bucco-lingual alveolar width and compromise the placement of implants in the ideal location or prevent placement altogether. After initial healing, these sites will require block or other types of onlay grafting to restore the patient to proper form and function before implant placement and/or prosthetic rehabilitation can ensue. Practitioners who are early in their learning curve and are not experienced with the types of reparative steps required to treat these and other potential complications should avoid these more challenging extractions.
Methods and Materials
When multi-rooted teeth are in need of removal, the first step should be to remove the crown and section the roots. This is usually accomplished with a conventional high-speed handpiece and carbide fissure bur. At this point, the roots can be treated just as single-rooted teeth and be removed using the same armamentarium.
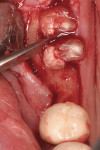
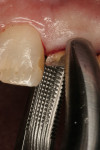
The first instruments used in this part of the process are thin-bladed hand instruments. Depending on the manufacturer, they are called periotomes (Figure 4) or lexi-cuts. Their purpose is to separate the circumferential and trans-gingival fibers from their cemental attachment. After this stage, the same instruments plus luxators (Figure 5) or lexits are inserted into the periodontal ligament (PDL) space. At this time, the periodontal ligament fibers are going to be stretched and broken. This is the point of the procedure that must be performed slowly and carefully. When there are curved, thin, non-vital, or bulbous roots, significant mobility of each should be achieved before attempted extraction from the socket.
When there is still minimal or no mobility of the individual roots, the operator faces a number of choices. One option is to take a long shank, fine diamond, or carbide bur and place it in the periodontal ligament (PDL) space. Another possibility is to use an ultrasonic bone surgery device (Piezosurgery® Incorporated, https://www.piezosurgery.us) (Figure 6) or powered periotome (Westport Medical, https://www.westportmedical.com) (Figure 7) in the same area and in a similar manner. When using the piezoelectric surgery device, the irrigant is chilled saline. This maintains the sterility of the site and decreases the chance of overheating the bone. Additionally, there is less chance of damaging bone compared to using a high-speed handpiece. With a conventional high-speed, there are a few potential complications. Cooling the site is usually done with normal tap water. This may not be sterile6 and is not chilled. Potential issues include infection, bone overheating, and a spray of air-water-bacteria mix over a much larger area. An air embolism may result from inadvertent entrance into a fascial plane.7 With a rotating bur, there is a great chance of removing bone, not just dentin, during the extraction procedure.
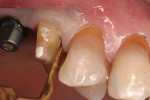
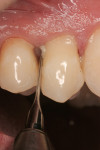
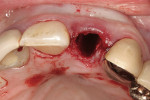
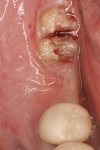
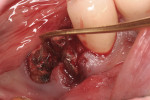
After initial mobility has been achieved, and the periodontal ligament has been disassociated from the tooth to a great degree, the teeth or individual roots can be removed with minimal to no trauma to the bone or gingiva (Figure 8 and Figure 9). If there are only single roots or single-rooted teeth remaining, they are removed with rotational movement (Figure 10 and Figure 11) using forceps with great retention to small pieces of dentin.
Discussion
Numerous studies have shown the extent of vertical and/or horizontal site collapse after tooth extraction when no socket preservation/augmentation procedures have been performed. In their study, Dr. Iasella and coworkers compared extraction with no bone replacement to grafting and the insertion of a mineralized allograft.8 In the non-treated sites, the average socket decreased in bucco-lingual width from 9.1 mm to 6.4 mm. When a graft material was placed in the area at the time of extraction, on average the loss of bucco-lingual dimension was only 1.2 mm. The difference between the alterations was statistically and clinically significant.
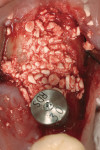
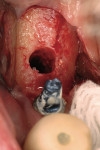
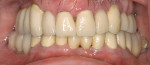
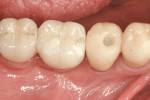
Use of the appropriate graft materials and techniques also enables true biologic regeneration in the site, not just fill. In their study, Drs. Vance, Greenwell, and coworkers9 compared volume preservation and vital bone formation in extraction sockets. Similar preservation of alveolar width and height was the result of the use of either bone replacement graft material. However, there was a significant difference histologically between the two procedures. In the sockets where an anorganic bovine replacement graft material was inserted, there was, on average, 26% vital bone formed with a tremendous variation in that amount (a standard deviation of 20% was found). Where allograft bone mixed with calcium sulfate and a binder was placed, there was 61% vital bone formed on a more consistent basis (a standard deviation of less than 10%). This was one of numerous articles questioning the short-term healing of xenograft in extraction sockets if one of the desired outcomes is vital bone formation in the area. Similar results have been seen clinically with the use of calcium sulfate alone (DentoGen, Orthogen, https://www.orthogencorp.com). Initially (Figure 12), there was a hopeless mandibular second molar with site collapse in the first molar site. After extraction of tooth No. 31, the site was grafted with Nanogen calcium sulfate pellets (Orthogen) mixed with DentoGen calcium sulfate (Figure 13) to regenerate the socket and augment the site of tooth No. 30. Reentry at 4 months shows a completely healed ridge with no evidence of graft particles and sufficient width to place a wide-diameter SybronPRO™ TL (Sybron Implants, sybronimplants.com) (Figure 14). After restoration, the facial view demonstrated significantly better esthetics than the site collapse evident on the contralateral side (Figure 15). When viewed from the occlusal, the crowns have normal anatomic size and sufficient keratinized tissue borders (Figure 16). Most importantly, the histologic evaluation demonstrates vital bone formation with no residual graft material in the site (Figure 17).
In 2004, Artzi and coworkers studied the healing patterns around inorganic bovine bone mineral and beta tricalcium phosphate in prepared defects.10 While, at 24 months postoperatively, areas treated with both graft materials healed well, there were significant differences histologically. There was still a significant presence of the anorganic bovine bone at the end of the study, whereas the tricalcium phosphate had fully resorbed. Three months after the grafting, a majority of the socket grafted with inorganic bovine bone mineral was filled with connective tissue and graft material. Only vital bone had incorporated particles of the graft at the edges of the defect. Most of the tricalcium phosphate had resorbed and been replaced by vital bone at the same time frame. The authors concluded histologically that there was delayed socket healing at 3 months and no resorption of the inorganic bovine bone mineral after the 6-month time period. Using pure phase beta tricalcium phosphate has been shown in humans to both preserve bone volume and result in vital bone formation.11 In this prospective study on extraction socket healing, grafting with a tricalcium phosphate and covering the site with a barrier preserved 89% of the socket width. Histologic evaluation of some of the sockets corroborated graft resorption and vital bone replacement in the time frame desired for placement of dental implants.
The insertion of an immediate socket implant with conventional surgical procedures has not shown predictable maintenance of the bucco-lingual socket width. The research performed by Botticelli and coworkers12 demonstrated both buccal and palatal or lingual bone loss after immediate-socket implants were placed. In their study, there was a 56% loss of width from the buccal bone resorption as well as 30% from the lingual side. Although, clinically, there appeared to be bone next to the buccal surface of the implant, they concluded that this was new bone formation inside the socket after significant buccal and palatal bony ridge resorption. Additionally, there was no implant retrieval performed to enable the potential verification of osseointegration in the defect site. Schropp and coworkers13 investigated healing of the infrabony defects around immediate-socket implants. They found that infrabony defects healed in the range of 39% to 75%. Where dehiscences were present, these areas only healed 25%. There was no predictability as far as complete healing in any of the infrabony spaces around immediate-socket implants. Additionally, the survival rate of implants placed in a delayed manner was 96% compared to only 91% in the immediate-socket sites.
As the “need” of patients to have dental implants placed and restored more quickly pervades the dental field,14 implant surfaces, prosthetic interfaces, and the ability to maximize vital bone formation and contact in the socket area must keep pace with the increasing challenges placed on dental implants. Having more dense bone in the receptor site and more vital bone may be factors in maintaining the longevity of implants in function. This may occur through increasing the percentage of bone-to-implant contact, which will assist in secondary stabilization of the inserted implant. This has been discussed in multiple research papers discussing potential reasons for dental implant failure,15 which consistently include poor bone quality.16
Formation of denser receptor sites filled with vital bone should decrease or eliminate this potential cause of loss of dental implants. Maintaining the full height and width of alveolar bone at the time of extraction assists in maximizing bone and keratinized gingiva formation. As we approach 100 years of scientifically documented use of dental implants for prosthetic retention,17 optimal functional and esthetic results are desired, and atraumatic extraction and predictable bone regeneration are the basic steps to making an ideal foundation for osseointegration.
Conclusion
The surgeon has the ability, and prerogative, to accomplish many goals when teeth are extracted. Minimizing surgical trauma to the patient ensures that future procedures will not be declined on an emotional basis. Proper extraction technique will preserve the buccal plate of bone, increasing bone fill in sockets with or without the placement of an immediate implant. Many techniques and materials used after extraction will lead to volume preservation of hard and soft tissue. Proper choice of bone replacement graft and barrier materials will give the patient the optimal biologic, regenerative result. Combining the ideal techniques and products will maximize the biologic processes and should enable success rates of dental implants placed in these sites to approach and stay near 100%.
Disclosure
Dr. Horowitz has received Grant/Research support from Sybron Implants. He has received honoraria from Sybron Implants and Osteogenics, and is a current consultant for Sybron Implants, Osteogenics, and Orthogen.
References
1. Linkow LI. Implants for edentulous arches. In: Winkler S. ed. Essentials of Complete Denture Prosthodontics. 1st ed. Philadelphia, Pa: WB Saunders Co; 1979;633-694.
2. Branemark PI, Adell R, Breine U, et al. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scandinavian Journal of Plastic and Reconstructive Surgery. 1969;3(2):81-100.
3. Mazor Z, Horowitz R, DelCorso M, et al. Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole grafting material: A radiologic and histologic study at 6 months. J Perio. 2009;80(12):2056-2064.
4. Jaffin RA, Berman CL. The excessive loss of Branemark fixtures in type IV bone: a 5-year analysis. J Perio. 1991;62(1):2-4.
5. Jung UW, Choi JY, Kim CS, et al. Evaluation of mandibular posterior single implants with two different surfaces: a 5-year comparative study. J Perio. 2008;79(10):1857-1863.
6. Frosina CJ, Mathews E. How is your water? An interdisciplinary approach to management of biofilm in dental unit waterlines. Probe. 1999;33(3):15-21.
7. Ali A, Cunliffe DR, Watt-Smith SR. Surgical emphysema and pneumomediastinum complicating dental extraction. Br Dent J. 2000;188:589-590.
8. Iasella J, Greenwell H, Miller R, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Perio. 2003;74(7):990-999.
9. Vance GS, Greenwell H, Miller RL, et al. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2004;19(4):491-497.
10. Artzi Z, Weinreb M, Givol N, et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: a 24-month longitudinal histologic study and morphometric analysis. Int J Oral Maxillofac Implants. 2004;19(3):357-368.
11. Horowitz R, Mazor Z, Miller R, et al. Clinical evaluation of alveolar ridge preservation with a beta-tricalcium phosphate socket graft. Comp Cont Educ Dent. 2009;30(9):2-12.
12. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction site. J Clin Perio. 2004;13(10):820-828.
13. Schropp L, Kostopoulos L, Wenzel A. Bone healing following immediate versus delayed placement of titanium implants into extraction sockets: a prospective clinical study. Int J Oral Maxillofac Implants. 2003;18(2): 189-199.
14. Linkow LI. Miller RJ. Immediate loading of endosseous implants is not new. J Oral Implant. 2004:30(5):314-317.
15. Esposito M, Hirsch J, Lekholm U, et al. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: A review of the literature. Int J Oral Maxillofac Implants. 1999;14:473-490.
16. Iezzi G, Degidi M, Scarano A, et al. Bone response to submerged, unloaded implants inserted in poor bone sites: a histological and histomorphometrical study of 8 titanium implants retrieved from man.J Oral Implant. 2005;31(5):225-233.
17. Greenfield EJ. Implantation of artificial crown and bridge abutments. Dental Cosmos. 1913;55:364-369, 430-439.
About the Authors
Robert A. Horowitz, DDS
Assistant Clinical Professor in Implant Dentistry and Periodontics
College of Dentistry
New York University
New York, New York
Private Practice in Periodontics and Implant Dentistry
Scarsdale, New York
Ziv Mazor, DMD
President Elect
Israeli Association of Oral Implants
Past President
Israeli Periodontal Society
Private Practice in Periodontics and Implant Dentistry
Raanana, Israel



 http://www.ckdental.net
http://www.ckdental.net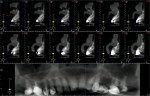
 http://www.angelfire.com/ky2/PSTDental
http://www.angelfire.com/ky2/PSTDental