The Art and Science of Tooth Whitening
Gerard Kugel, DMD, MS, PhD; Susana Ferreira, DDS
The dental profession has succeeded in reducing caries and periodontal diseases and, as a result, dental problems have decreased to the point where esthetic improvements are now more attainable.1 One of the fastest growing areas of esthetic dentistry today is the management of the discolored and hypoplastic dentition. The demand for an improved appearance and a whiter smile has made tooth whitening a very popular dental procedure. Tooth whitening, sometimes referred to as “bleaching,” offers a conservative treatment option for discolored teeth in comparison with resin-bonded composites, porcelain veneers, or crowns.2,3
Successful whitening treatment depends on type, intensity, and location of the discoloration and a careful diagnosis by the practitioner. Tooth discolorations can be superficial changes that affect the enamel surface, or can be deeper staining that affects the entire tooth structure. It is essential for the dentist to identify the type of discoloration in the patient’s tooth, diagnose the cause, and define the appropriate treatment plan.
Discoloration in vital teeth can result from aging, tobacco use, chromogenic foods, medications, and pulpal pathology.4 More severe discoloration results from systematically prescribed medication during tooth formation, excessive fluoride intake during enamel formation and calcification, some systemic conditions, dental conditions and/or treatment, and aging. Tetracycline discoloration occurs with the systemic intake of tetracycline during tooth formation (the second trimester in utero to 8 years of age).
Various systemic conditions can also cause tooth discolorations. Amelogenesis imperfecta may result in hypoplasia or hypocalcification with yellow or brown stains, and dentinogenesis imperfecta may result in brownish-violet, yellowish, or gray discolorations.5 Hypoplasia or hypocalcification can occur with clefting of the lip and palate or with acquired illnesses such as cerebral palsy, serious renal damage, and severe allergies.6 Blue, brownish, or green tooth discolorations may be caused by the destruction of an excessive number of blood-cell erythrocytes in erythroblastosis fetalis, which is a result of Rh-factor incompatibility between the mother and fetus.5,6
Patients with porphyria, a rare condition that causes an excessive production of pigment, may result in a red, purplish-brown, or brownish tooth discoloration.6-8 Dental caries, discolored acrylic or restorations (made of composite, amalgam, and/or metal), pins, posts, and other materials used in the dental environment can also have an adverse effect on tooth coloration, resulting in a darkened shade of the patient’s dentition.
Aging usually brings thinning of the enamel, loss of the translucent enamel layer, and formation of secondary dentin. The combination of less enamel and darker, opaque dentin creates an older-looking, darker tooth.6
Attempts to find an effective whitening method have been made throughout the history of dentistry. Early studies reported the combination use of pyrozone 25% and electricity to whiten endodontically treated teeth.9 Another study reported the use of hypochloric acid to treat endemic fluorosis in 1916.10 In the late 1930s, professionals advocated the use of 30% hydrogen peroxide, ether, and heat to treat fluorosis staining.11 In 1966, as a result of chronic endemic dental fluorosis, the combination use of hydrochloric acid and hydrogen peroxide was promoted to remove “brown stain from mottled teeth.”12 The early efforts to whiten teeth relied on the assumption that the process involved the removal of extrinsic enamel stain. The mechanism of action was poorly understood.
Unlike other previous studies, in 1970 Cohen and Parkins first published a method for whitening the discolored dentin of young adults with cystic fibrosis who had undergone tetracycline treatment.13 This publication indicated chemical penetration to the dentin using hydrogen peroxide to whiten the teeth. In 1976, Nutting and Poe introduced the walking bleach technique, which used 35% hydrogen peroxide and sodium perborate for whitening nonvital teeth.14 The breakthrough in tooth whitening was in 1989 when Haywood and Heymann developed the nightguard vital bleaching technique, in which carbamide peroxide gel was placed in a tray that the patient wore at home.15 This procedure is still widely used in the dental community as the take-home whitening system.
Tooth Whitening Systems
Tooth whitening with various concentrations of peroxide has been demonstrated to be safe and effective in a variety of regimens, including in-office procedures, dentist-prescribed and supervised home treatments, and over-the-counter (OTC) systems.16 Most dental practices in the United States offer some form of tooth whitening.
Take-Home Systems
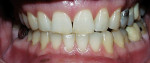
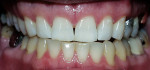
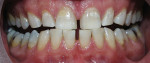
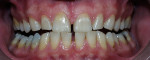
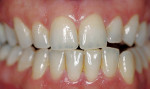
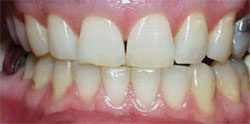
The most common regimen is the at-home use of a whitening agent for 2 weeks to 4 weeks, based on the color of the teeth at the start of treatment.15,17 With these systems, a tray is fabricated from a model of the patient’s teeth using a soft plastic nightguard. This tray is then loaded with carbamide peroxide gel and seated over the teeth for approximately 2 hours. The most commonly prescribed concentrations are 10% to 22% carbamide peroxide (Figure 1A and Figure 1B).
The advantage of the take-home systems is decreased cost when compared with in-office whitening options. The major disadvantages associated with take-home systems are that they require significant patient compliance concerning the number of applications, which usually involve 1 to 2 hours twice a day or overnight wear, and the entire treatment usually takes at least 4 weeks.
In-Office Systems
The most acceptable whitening applications are in-office techniques, dentist-prescribed take-home systems, or a com-bination of both. In-office whitening techniques generally use a 15%, 30%, or 35% hydrogen peroxide whitening agent (heated or nonheated).
The advantages of the in-office procedure are that it requires minimal patient compliance and offers immediate results. The disadvantages to this treatment are the chairtime required and the cost to the patient. This procedure also usually requires multiple office visits.
Combination Technique
Combining the two techniques (in-office and take-home) results in a reduction in the amount of time required and the need for repeated office visits as well as the expense associated with in-office whitening as a stand-alone technique.18 At the same time, the combined technique increases overall success and patient satisfaction. This procedure involves the use of a high concentration of hydrogen peroxide (35%) delivered chairside for 1 hour, followed by a take-home regimen of 5 days. This is often followed by an additional chairside application.19
Whitening Strips
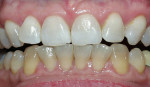
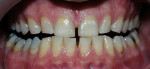
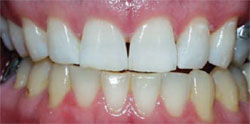
This at-home method involves using a 5.3% hydrogen peroxide-impregnated polyethylene strip (Crest®Whitestrips®, Procter & Gamble, Cincinnati, OH ) for 30 minutes twice daily (Figure 2). The whitening strip method is recommended for maintaining already whitened teeth, but it can also be a good option for patients who cannot afford the cost of other whitening treatments or who do not have the time for multiple dental visits for tray fabrication.17
Light-Activated Whitening Systems
The high demand for “up-to-date” dental offices by patients and clinicians stimulated manufacturers to inundate the dental profession with advertisements empha-sizing that light-activated tooth whitening is the state of the art and should be part of the armamentarium of the office, without clear evidence of its usefulness. The introduction of light-activated devices, such as plasma arc, light-emitting diodes, argon lasers, and metal halide and xenon-halogen lights by dental manufacturers has helped to create a new category of whitening systems.
To gain treatment time, clinicians have attempted to accelerate the degradation of hydrogen peroxide by using light or heat.20 In 2002, controversial articles were published that evaluated the efficacy of light-activated whitening agents. In one article, positive results using light-activated systems were reported,21 while other articles concluded the opposite findings.22,23 These articles either maintained24 or questioned the results.25 Cohen and Parkins introduced a technique for whitening discolored teeth using hydrogen peroxide and a handheld heating source.13
The assumption behind the use of light or heat is that clinically tolerable levels of heat will speed the breakdown of hydrogen peroxide tooth-whitening chemicals, and this accelerated breakdown causes teeth to lighten more rapidly per unit time. In a recent study, the decomposition of hydrogen peroxide was measured by the amount of oxygen released. The data from this study indicated that at temperatures of up to 85° C, accelerated decomposition of the 35% hydrogen peroxide was minimal when compared to the decomposition of the control gel.22
A recent in vitro study has shown that the use of intense lights does elevate temperature of the whitening material and, as a result, causes an increase in intrapulpal temperature. This may have an impact on post-whitening tooth sensitivity and pulpal health.26 Another in vitro research article has verified that the use of laser-activated hydrogen peroxide does not produce any perceivable color change.24
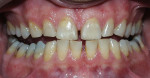
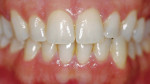
Light-activated chairside whitening systems are believed to offer the benefits of being less time-consuming while producing faster results. The use of light-activated whitening systems to accelerate the whitening process is still in question, and more evidence needs to be gathered to make a more precise assertion of its effectiveness (Figure 3A; Figure 3B; Figure 3C; Figure 3D).
Toothpaste
Many types of toothpaste are marketed as whitening products, but only a small number of them show stain removal ability and effectiveness. Most of these toothpastes do not contain whitening agents in their formulation. The ones that do have a very low bleach concentration and the contact time is too short to be effective.27 These whitening toothpastes contain mild abrasives to remove surface stains, and the peroxide content in the toothpastes are very low (≤ 1%). The exposure of the toothpastes on the tooth is minimal; therefore, any whitening is also minimal.28
Over-the-Counter Products
Easy availability of OTC whitening products has made whitening of teeth more popular among people of all ages. There are different types of OTC products, such as whitening dentifrices, trays, whitening strips, paint-on brush applications, and light-activated tooth-whitening systems (Figure 4A and Figure 4B). An OTC whitening kit requires the consumer either to use a prefabricated tray or fabricate a semimolded tray, and then fill it with the supplied whitening agents.
These types of at-home whitening systems are less than ideal because the trays are not custom-fitted and the formulation is often not as sophisticated as those dispensed by the dentist.29 It is important that clinicians discuss the use of OTC products with patients. If a patient uses an OTC product during dental treatment, it could affect final color matching by the dentist and/or the dentist’s ability to bond to recently whitened teeth.
Mechanism of action
The exact mechanism of action for tooth whitening is not completely understood. Hydrogen peroxide diffuses through the organic matrix of the enamel anddentin30-32 and then ionizes to initiate a redox chemical reaction. Hydrogen peroxide is an oxidizing agent, which breaks down to produce free radicals. Because the radicals have unpaired electrons, they are extremely electrophylic and unstable, and will attack most other organic molecules to achieve stability, generating other radicals. These radicals can react with most unsaturated bonds, resulting in disruption of electron conjugation and a change in the absorption energy of the organic molecules in tooth enamel. Simpler molecules that reflect less light are formed, creating a successful whitening action. This process occurs when the oxidizing agent (hydrogen peroxide) reacts with organic material in the spaces between the inorganic salts in tooth enamel.6
Whitening Penetration
Enamel is the densest tissue of the body. A 1951 study showed that radioisotope-labeled hydrogen peroxide penetrated through enamel to dentin and diffused into the pulp.33 Carbamide peroxide breaks down into hydrogen peroxide, carbon dioxide urea, and ammonia. Some of the by-products penetrate the dentinal tubules reaching the pulp, causing reversible pulpitis.30,34 Some of the pulpal enzymes are sensitive to hydrogen peroxide along with heat; however, in vital bleaching the amount of peroxide reaching the pulp compared to the amount of hydrogen peroxide required to cause this damage is very low.31 Gokay and colleagues showed that a higher peroxide diffusion was seen in teeth with restorations when a high concentration of carbamide peroxide was used as compared with the use of a lower concentration.34 In vitro data using extracted human teeth have demonstrated that a 10% carbamide peroxide gel can change the color of dentin over time.35
Side Effects
Toxicology
The US Food and Drug Administration approved hydrogen peroxide and carbamide peroxide to be used for debriding oral wounds. Although whitening is a longer process than debridment, in vitro toxicological evaluation of whitening agents, such as 10% carbamide peroxide or 4% hydrogen peroxide, showed fewer or comparable side effects of commonly used dental materials such as eugenol, dentifrices, mouthrinses, and composites.16
Studies have shown that daily exposure of carbamide peroxide should not exceed 10 mg/kg.36 Li and Matis found that the average amount of whitening agent used is 502 mg per application. Even if the patient swallowed all of the gel, it would not exceed more than 8.37 mg/kg.16 There have been no credible studies in animals or humans that link tooth whitening to issues of oral cancer; therefore, the safety factor of whitening agents is high.
Oral Side Effects
Mucosal irritation occurs mostly as a result of ill-fitting trays, improper application of the whitening gel, or longer-than-prescribed use. Schulte and colleagues showed that subjects with overnight exposure to whitening gel experienced mucosal irritation.37 Soft-tissue irritation generally is minimal, and is resolved either by adjustment of the whitening tray or shortly after cessation of the treatment.38 The thickness of the tray material (0.040 inches is recommended) is also important to prevent patient discomfort and other problems associated with the temporomandibular joint. If the whitening tray is fabricated from a thick material, the appliance can produce minor orthodontic movements.39
Tooth Sensitivity
Studies have shown that sensitivity occurs in 55% to 75% of the treatment groups, compared with 20% to 30% of the placebo groups. One study reported tooth sensitivity in approximately 15% of patients wearing only the whitening tray.40,41 The development of tooth sensitivity can be a multilateral phenomenon, caused by an allergic or chemical sensitivity to the composition of the tray or the whitening gel or the free radical formation of the gel.42 It can also result from overzealous toothbrushing during participation in a clinical trial.41 Glycerin, which is used to carry the active ingredient, can absorb water and thereby cause a dehydration effect, also resulting in sensitivity.39,42
Sensitivity as a result of tooth whitening is generally mild and transient. It occurs earlier in treatment and decreases as treatment continues. Occurrence might be a result of the close contact of gel to the tooth and disappearance may be a result of sensory accommodation.41 Tooth sensitivity and gingival irritation are always concerns when tooth whitening is performed. Both in-office and take-home whitening procedures have been reported to induce sensitivity in a significant number of patients,43 but tooth whitening is generally well tolerated.
CONCLUSION
Although tooth whitening is one of the most popular dental procedures, it is also one of the least understood. The mechanism of action is still unclear. There is little data as to the effects of both concentration and dose on outcome. The techniques for measuring color change have been brought into question, and the causes of sensitivity and the effects of long-term exposure to hydrogen peroxide are also not clear. The issue of rebound in color has not been well examined, and issues related to maintenance of the whitening effect are also poorly understood.
The immediate placement of composite resin on whitened teeth has been controversial. According to a study evaluating the shear bond strength of composite restorations placed on whitened and nonwhitened teeth, there was no statistically significant difference when the composites were placed at 24 hours, 48 hours, 4 days, or 6 days.44 A different study, which evaluated the effects of take-home whitening systems on enamel surfaces, suggested that a period of 4 days must elapse before bonding to a tooth whitened with a peroxide material, while no delay is necessary for a non-peroxide�based whitening system.45
Most recently, there has been a push to find ways to accelerate and improve the delivery of the whitening process. These include the application of a number of different light sources believed to accelerate the breakdown of peroxide and thus speed up the whitening process. However, the research in this area has been controversial, with publications having quite different conclusions as to the efficacy of light-activated whitening.
Finally, the issues of adverse events and possible side effects have been reviewed. The toxicological side effects of tooth whitening seem to be minimal. However, tooth sensitivity can be quite significant. Although its causes are poorly understood, tooth sensitivity is most often seen as the result of tooth dehydration.
While patient demand for tooth whitening is at an all-time high, and dentists have more options of treatment, it is important that dentists evaluate which option is most ideal for each patient, factoring in the patient’s cost and time issues as well as sensitivity to the procedure.
Acknowledgment
Previously published in Journal Mass Dental Society. 2005;53(4):34-37.
References
1. Burrell KH. ADA supports vital tooth bleaching—but look for the seal. J Am Dent Assoc. 1997;128(suppl):3S-5S.
2. Papathanasiou A, Bardwell D, Kugel G. Combining in office and take home whitening systems. Contemp Esthet Restorative Pract. 2000;4(8):88-91.
3. Papathanasiou A, Bardwell D, Kugel G. A clinical study evaluating a new chairside and take home whitening system. Compend Contin Educ Dent. 2001;22(4):289-298.
4. Blankenau R, Goldstein RE, Haywood VB. The current status of vital tooth whitening techniques. Compend Contin Educ Dent. 1999;20(8):781-788.
5. Shafer WG, Hine MK, Levy BL. Oral Pathology. 3rd ed. Philadelphia, PA: WB Saunders; 1974.
6. Goldstein RE, Garber DA. Complete Dental Bleaching. Chicago, IL: Quintessence; 1995.
7. Jordan RE, Boksman L. Conservative vital bleaching treatment of discolored dentition. Compend Contin Educ Dent. 1984;5(10):803-807.
8. Faunce F. Management of discolored teeth. Dent Clin North Am. 1983;27(4): 657-670.
9. Westlake A. Bleaching teeth by electricity. Am J Dent Sci. 1895;29:101.
10. Adams TC. Enamel color modifications by controlled hydrochloric acid pumice abrasion: a review with case summaries. J Indiana Dent Assoc. 1987;66(5):23-26.
11. Younger HB. Bleaching fluorine stain from mottled enamel. Texas Dent J. 1939;57:380.
12. McInnes J. Removing brown stain from teeth. Ariz Dent J. 1966;12(4):13-15.
13. Cohen S, Parkins FM. Bleaching tetracycline-stained vital teeth. Oral Surg Oral Med Oral Path. 1970;29(3):465-471.
14. Nutting EB, Poe GS. Chemical bleaching of discolored endodontically treated teeth. Dent Clin North Am. 1967;10:655-662.
15. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20(3):173-176.
16. Yiming Li. Toxicological considerations of tooth bleaching using peroxide containing agents. J Am Dent Assoc. 1997;128(Suppl): 31S-36S.
17. Kugel G. Nontray whitening. Compend Contin Educ Dent. 2000;21(6):524-528.
18. Garber DA. Dentist-monitored bleaching: a discussion of combination and laser bleaching. J Am Dent Assoc. 1997;128(suppl):26S-30S.
19. Kugel G, Perry RD, Hoang E, et al. Effective tooth bleaching in 5 days: using a combined in-office and at-home bleaching system. Compend Contin Educ Dent. 1997;18(4): 378-383.
20. Goldstein RE. Esthetics in Dentistry-Principles, Communications, Treatment Methods. 2nd ed; vol 1. Hamilton-London: BC Becker-Year Book; 1998. p 245.
21. Tavares M, Stultz J, Newman M, et al. Light augments tooth whitening with peroxide. J Am Dent Assoc. 2003;134(2):167-175.
22. Hein DK, Ploeger BJ, Hartup JK, et al. In-office vital tooth bleaching—what do lights add? Compend Contin Educ Dent. 2003;24(4A): 340-352.
23. Papathanasiou A, Kastali S, Perry RD, et al. Clinical evaluation of a 35% hydrogen peroxide in-office whitening system. Compend Contin Educ Dent. 2002;23(4): 335-338.
24. Jones AH, Diaz-Arnold AM, Vargas MA, et al. Colorimetric assessment of laser and home bleaching techniques. J Esthet Dent. 1999;11(2): 87-94.
25. ADA Council on Scientific Affairs. Laser-assisted bleaching: an update. J Am Dent Assoc. 1998;129(10):1484-1487.
26. Baik JW, Rueggeberg FA, Liewehr FR. Effect of light-enhanced bleaching on in vitro surface and intrapulpal temperature rise. J Esthet Restorative Dent. 2001;13(6):370-378.
27. Sharif N, MacDonald E, Hughes J, et al. The chemical stain removal properties of “whitening” toothpaste products: studies in vitro. Br Dent J. 2000:188(11):620-624.
28. Donly KJ, Donly AS, Baharloo L, et al. Tooth whitening in children. Compend Contin Educ Dent. 2002;23(1A):22-28.
29. Kugel G. Over-the-counter tooth-whitening systems. Compend Contin Educ Dent. 2003;24(4A): 376-383.
30. Bowles WH, Ugwuneri Z. Pulp chamber penetration by hydrogen peroxide following vital bleaching procedures. J Endod. 1987;13(8): 375-377.
31. Bowles WH, Thompson LR. Vital bleaching: the effect of heat and hydrogen peroxide on pulpal enzymes. J Endod. 1986; 12(3):108-112.
32. Fuss Z, Szajkis S, Tagger M. Tublar permeability to calcium hydroxide and to bleaching agents. J Endod. 1989;15(8):362-364.
33. Bartelstone HJ. Radioiodine penetration through intact enamel with uptake by bloodstream and thyroid gland. J Dent Res. 1951;30(5):728-733.
34. Gokay O, Tuncbilek M, Ertan R. Penetration of the pulp chamber by carbamide peroxide bleaching agents on teeth restored with composite resin. J Oral Rehabil. 2000;27(5):428-431.
35. McCaslin AJ, Haywood VB, Potter BJ, et al. Assessing dentin color changes from nightguard vital bleaching. J Am Dent Assoc. 1999,130(10): 1485-1490.
36. Dahl JE, Becher R. Acute toxicity of carbamide peroxide and commercially available tooth-bleaching agent in rats. J Dent Res. 1995;74(2):710-714.
37. Schulte J, Morrissette DB, Gasior EJ, et al. Clinical changes on the gingiva as a result of at-home bleaching. Compend Contin Educ Dent. 1993;14(11): 1362-1366.
38. Haywood VB, Leonard RH, Chauncy NF, et al. Effectiveness, side effects and long-term status of nightguard vital bleaching. J Am Dent Assoc. 1994;125(9):1219-1226.
39. Pohjola R, Browning WD, Hackman ST, et al. Sensitivity and tooth whitening agents. J Esthet Restorative Dent. 2002;14(2): 85-91.
40. Haywood VB, Caughman FW, Frazier KB, et al. Tray delivery of potassium nitrate-fluoride to reduce bleaching sensitivity. Quintessence Int. 2001;32(2): 105-109.
41. Jorgensen MG, Carroll WB. Incidence of tooth sensitivity after home whitening treatment. J Am Dent Assoc. 2002;133(8): 1076-1082.
42. Leonard RH Jr., Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital bleaching. Quintessence Int. 1997;28(8): 527-534.
43. Nathanson D. Vital tooth bleaching: sensitivity and pulpal considerations. J Am Dent Assoc. 1997;128(suppl):41S-44S.
44. Yu P, Aboushala A, Bardwell D. Effect of bleaching on composite resin shear bond strength in vitro. J Dent Res. 1999;78 (spec iss). Abstract 1445.
45. MacKay M, Perry R, Swift E, et al. Effects of the two home bleaching systems on enamel surfaces. J Dent Res. 1997;76(spec iss). Abstract 1405.
 |
 |
|
| Figure 1A Pretreatment view using a 15% carbamide peroxide take-home whitening system. | Figure 1B Posttreatment view using a 15% carbamide peroxide take-home whitening system. | |
 |
 |
|
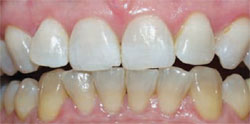
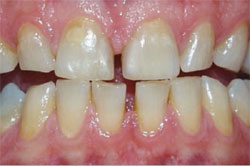
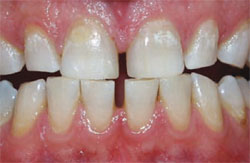
| Figure 2 Crest® Whitestrips® used for tetracyclinestained teeth. The lower arch was used as a control. | Figure 3A Pretreatment view of a light-activated whitening system. | |
 |
 |
|
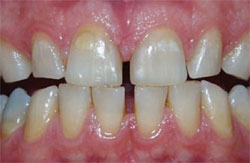
| Figure 3B Both arches immediately posttreatment using a light-activated whitening system. | Figure 3C Both arches at the 7-day recall using a light-activated whitening system. | |
 |
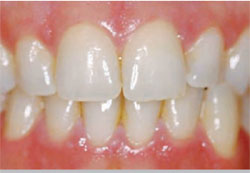 |
|
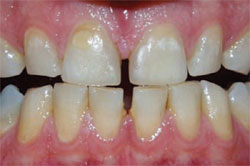
| Figure 3D Both arches at the 30-day recall using a light-activated whitening system. | Figure 4A Pretreatment view using an OTC, light-activated tooth whitening system. | |
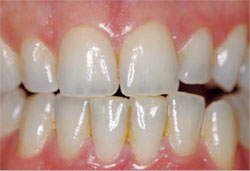 |
||
| Figure 4B Posttreatment view using an OTC, light-activated tooth whitening system. | ||
| About the Authors | ||
 Gerard Kugel, DMD, MS, PhD Gerard Kugel, DMD, MS, PhDProfessor Associate Dean for Research Tufts University School of Dental Medicine Boston, Massachusetts |
||
 Susana Ferreira, DDS Susana Ferreira, DDSAssistant Professor Department of Prosthodontics and Operative Dentistry Tufts University School of Dental Medicine Boston, Massachusetts |
||