
Henry Schein, Inc. (Nasdaq: HSIC), the world’s largest provider of health care solutions to office-based dental and medical practitioners, announced today that it has acquired a 70% ownership position in eAssist Dental Solutions (eAssist), the developer of a leading and fast-growing virtual dental billing outsourcing service that will advance the Company's mission to offer best-of-breed solutions to help dental practices operate more efficiently and profitably, freeing up practice resources to focus on patient care.
Headquartered in American Fork, UT, eAssist was founded in 2011 by James Anderson, DMD, and Sandy Odle to assist with billing challenges in his dental practices. Dr. Anderson will continue to manage the eAssist business as Chief Executive Officer, along with the current eAssist management team. In 2020, eAssist had sales of approximately $31 million. Henry Schein expects that eAssist will be slightly dilutive to the Company’s 2021 financial results and to be accretive thereafter. Financial terms were not disclosed.
“Henry Schein has a long-standing commitment to supporting dental practice growth, efficiency, and profitability through our comprehensive offering of solutions,” said Stanley M. Bergman, Chairman of the Board and Chief Executive Officer of Henry Schein. “We are delighted to add eAssist to our leading portfolio of value-added services, which will help our customers improve their critical insurance and billing functions so dental teams can focus on delivering quality patient care. We welcome our new colleagues to Team Schein and look forward to continued success together.”
eAssist uses a combination of technology and human expertise to provide outsourced insurance billing services to dental practices, helping practices to free-up resources to focus on patient care and clinical outcomes. By enhancing collections processes, eAssist allows dental practices to simplify operations and reduce stress related to cashflow.
“By addressing the important topic of collections, we not only expand our value-added services, but also strengthen our relationship as a trusted advisor to our customers,” said Jonathan Koch, Senior Vice President and CEO, Henry Schein Global Dental Group. “eAssist will help practices enhance cash flow from timely collections and more accurate claims submissions and processing.”
eAssist is currently designed to support dental practices of all sizes through technology supported by a network of dental billing professionals. In addition to its core dental billing service, newer offerings from eAssist include securing detailed patient eligibility information, billing certain dental procedures through medical insurance, and practice bookkeeping.
“Henry Schein represents an exceptional and complementary cultural fit as a partner for eAssist,” said Dr. Anderson. “Through new and powerful sales, marketing, and technology resources, we expect to bring the myriad of benefits from eAssist’s technology-powered service offerings to many more dental practices. Henry Schein has a clear commitment to advancing dental practice efficiency and profitability, and we believe they are the right partner to help us help even more dentists succeed.”
About Henry Schein, Inc.
Henry Schein, Inc. (Nasdaq: HSIC) is a solutions company for health care professionals powered by a network of people and technology. With more than 20,000 Team Schein Members worldwide, the Company's network of trusted advisors provides more than 1 million customers globally with more than 300 valued solutions that improve operational success and clinical outcomes. Our Business, Clinical, Technology, and Supply Chain solutions help office-based dental and medical practitioners work more efficiently so they can provide quality care more effectively. These solutions also support dental laboratories, government and institutional healthcare clinics, as well as other alternate care sites.
Henry Schein operates through a centralized and automated distribution network, with a selection of more than 120,000 branded products and Henry Schein private-brand products in stock, as well as more than 180,000 additional products available as special-order items.
A FORTUNE 500 Company and a member of the S&P 500® index, Henry Schein is headquartered in Melville, N.Y., and has operations or affiliates in 31 countries. The Company's sales from continuing operations reached $10.1 billion in 2020, and have grown at a compound annual rate of approximately 12 percent since Henry Schein became a public company in 1995.
For more information, visit Henry Schein at www.henryschein.com, Facebook.com/HenrySchein, and @HenrySchein on Twitter.
About eAssist Dental Solutions
eAssist Dental Solutions is the nation’s leading provider of remote dental billing and patient billing services for dental offices. The more than 1,200 eAssist team members serve over 2,000 dental practices through proprietary technology platforms that enhance a dental practice’s revenue cycle management. The company’s end-to-end solution eases the burden on office staff, ultimately helping practices be more efficient, profitable and patient-focused.
To learn more, visit eAssist online at: www.dentalbilling.com, Facebook.com/eAssistMe, and @eAssist.me on Instagram.
Cautionary Note Regarding Forward-Looking Statements
In accordance with the “Safe Harbor” provisions of the Private Securities Litigation Reform Act of 1995, we provide the following cautionary remarks regarding important factors that, among others, could cause future results to differ materially from the forward-looking statements, expectations and assumptions expressed or implied herein. All forward-looking statements made by us are subject to risks and uncertainties and are not guarantees of future performance. These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance, and achievements or industry results to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These statements are generally identified by the use of such terms as “may,” “could,” “expect,” “intend,” “believe,” “plan,” “estimate,” “forecast,” “project,” “anticipate,” “to be,” “to make,” or other comparable terms. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the documents we file with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K. Forward looking statements include the overall impact of the Novel Coronavirus Disease 2019 (COVID-19) on the Company, its results of operations, liquidity, and financial condition (including any estimates of the impact on these items), the rate and consistency with which dental and other practices resume or maintain normal operations in the United States and internationally, expectations regarding personal protective equipment (“PPE”) and COVID-19 related product sales and inventory levels and whether additional resurgences of the virus will adversely impact the resumption of normal operations, the impact of restructuring programs as well as of any announced and future acquisitions, and more generally current expectations regarding performance in current and future periods. Forward looking statements also include the (i) ability of the Company to make additional testing available, the nature of those tests and the number of tests intended to be made available and the timing for availability, the nature of the target market, as well as the efficacy or relative efficacy of the test results given that the test efficacy has not been, or will not have been, independently verified under normal FDA procedures and (ii) potential for the Company to distribute the COVID-19 vaccines and ancillary supplies.
Risk factors and uncertainties that could cause actual results to differ materially from current and historical results include, but are not limited to: risks associated with COVID-19, as well as other disease outbreaks, epidemics, pandemics, or similar wide spread public health concerns and other natural disasters or acts of terrorism; our dependence on third parties for the manufacture and supply of our products; our ability to develop or acquire and maintain and protect new products (particularly technology products) and technologies that achieve market acceptance with acceptable margins; transitional challenges associated with acquisitions, dispositions and joint ventures, including the failure to achieve anticipated synergies/benefits; financial and tax risks associated with acquisitions, dispositions and joint ventures; certain provisions in our governing documents that may discourage third-party acquisitions of us; effects of a highly competitive (including, without limitation, competition from third-party online commerce sites) and consolidating market; the potential repeal or judicial prohibition on implementation of the Affordable Care Act; changes in the health care industry; risks from expansion of customer purchasing power and multi-tiered costing structures; increases in shipping costs for our products or other service issues with our third-party shippers; general global macro-economic and political conditions, including international trade agreements and potential trade barriers; failure to comply with existing and future regulatory requirements; risks associated with the EU Medical Device Regulation; failure to comply with laws and regulations relating to health care fraud or other laws and regulations; failure to comply with laws and regulations relating to the confidentiality of sensitive personal information or standards in electronic health records or transmissions; changes in tax legislation; litigation risks; new or unanticipated litigation developments and the status of litigation matters; cyberattacks or other privacy or data security breaches; risks associated with our global operations; our dependence on our senior management, as well as employee hiring and retention; and disruptions in financial markets. The order in which these factors appear should not be construed to indicate their relative importance or priority.
We caution that these factors may not be exhaustive and that many of these factors are beyond our ability to control or predict. Accordingly, any forward-looking statements contained herein should not be relied upon as a prediction of actual results. We undertake no duty and have no obligation to update forward-looking statements.





 Sunbit has launched a buy now, pay later solution made specifically for dental practices. Many practices are selecting Sunbit as the primary buy now, pay later (BNPL) partner of choice, with its ability to serve 85% of patients who apply. Sunbit's BNPL program, made for dental, offers access to financing for procedures between $60 and $10,000, with 6, 12, 18 and 24 month payment plan options. All qualified patients get access to 0% for 6- or 12-month payment plans. Sunbit powers the application process and manages the collections process. The dental office gets paid right away, contributing directly to the cash flow of the business without the risk of a patient defaulting on the loan.
Sunbit has launched a buy now, pay later solution made specifically for dental practices. Many practices are selecting Sunbit as the primary buy now, pay later (BNPL) partner of choice, with its ability to serve 85% of patients who apply. Sunbit's BNPL program, made for dental, offers access to financing for procedures between $60 and $10,000, with 6, 12, 18 and 24 month payment plan options. All qualified patients get access to 0% for 6- or 12-month payment plans. Sunbit powers the application process and manages the collections process. The dental office gets paid right away, contributing directly to the cash flow of the business without the risk of a patient defaulting on the loan.
 MouthWatch, LLC a leader in innovative teledentistry solutions, digital case presentation tools and intraoral imaging devices, recently named Sylvia Rochon as its Senior Director of Sales and Marketing.
MouthWatch, LLC a leader in innovative teledentistry solutions, digital case presentation tools and intraoral imaging devices, recently named Sylvia Rochon as its Senior Director of Sales and Marketing.
 In a clearly distinctive move to make perfect smiles easy and accessible, Aspen Dental Management Inc. (ADMI) launches Motto, a new, clear aligner experience that does not force patients to choose between quality, affordability, convenience and expertise.
In a clearly distinctive move to make perfect smiles easy and accessible, Aspen Dental Management Inc. (ADMI) launches Motto, a new, clear aligner experience that does not force patients to choose between quality, affordability, convenience and expertise.
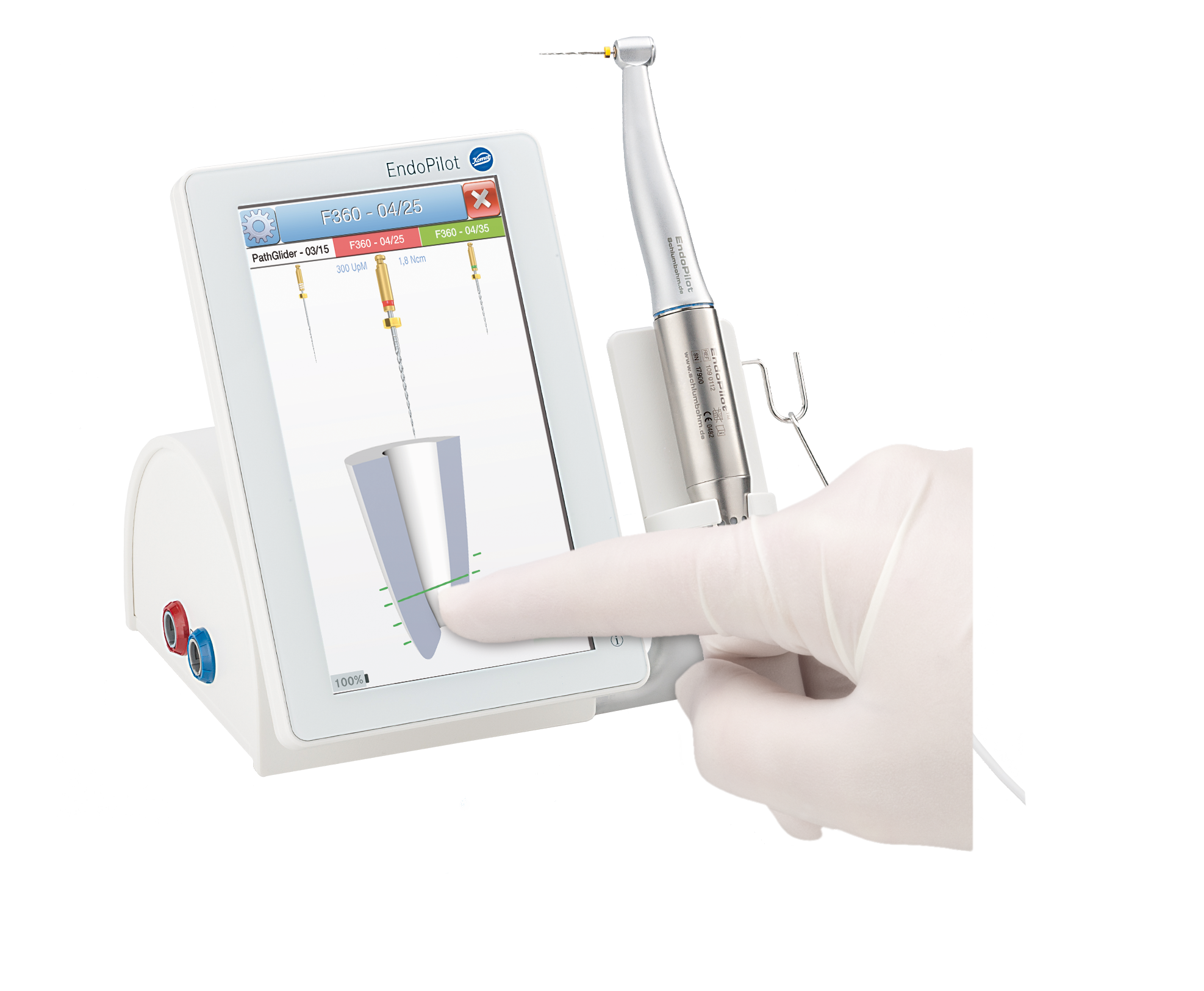 Komet USA today announced the availability of a new endodontic motor, EndoPilot. The EndoPilot is a powerful, torque and speed controlled endodontic motor with state-of-the-art technology that can help endodontists and general practitioners complete endodontic procedures more efficiently while ensuring patient safety.
Komet USA today announced the availability of a new endodontic motor, EndoPilot. The EndoPilot is a powerful, torque and speed controlled endodontic motor with state-of-the-art technology that can help endodontists and general practitioners complete endodontic procedures more efficiently while ensuring patient safety.
