

American Association of Endodontists members from Boston, Harvard and Tufts Dental Schools form trifecta of free care on “Teeth Worth Saving Day”
Yesterday, the American Association of Endodontists (AAE) provided more than 50 endodontic treatments (namely root canals) to patients in need in the Boston area. The services were given in collaboration with the endodontics departments at all three Boston dental schools: Boston University Henry M. Goldman School of Dental Medicine, Department of Endodontics; Harvard School of Dental Medicine, Division of Endodontics; and Tufts University School of Dental Medicine, Department of Endodontics.
As part of its Worth Saving campaign, which emphasizes the importance of saving natural treasures like your teeth, the AAE held a contest last March spotlighting four beautiful natural landmarks from around the country and asked Americans to vote on which one they felt was most “worth saving.” Boston’s Charles River Esplanade, preserved by the Esplanade Association, won the contest and with it a $20,000 donation from AAE, as well as $30,000 in donated endodontic care for Boston residents.
As home to more dental schools than any other U.S. city, all three Boston dental schools volunteered to provide the endodontic treatments, forming a trifecta of donated care just in time for the holidays. The treatments performed yesterday totaled more than $59,000 in value -- well over the amount promised.
“We’re thrilled to give back to the city of Boston and to collaborate with the endodontics departments of renowned dental schools in the area,” said Keith V. Krell, D.D.S., M.S., M.A., president of the AAE. “We are grateful for the schools’ generosity. And congratulations to the Charles River Esplanade for winning our contest and reminding us that there is so much worth saving – from natural landmarks to our natural teeth.”
Patients were pre-screened and pre-qualified based on need. From the Department of Endodontics at Tufts University School of Dental Medicine, a staff of 14 residents and three faculty participated in the initiative, treating 15 patients. Additionally, a team of three dental assistants and five administration staff led coordination efforts.
At Harvard University to Harvard School of Dental Medicine, Division of Endodontics, a staff of six residents performed treatments, along with two faculty and more than 10 dental student and first-year resident volunteers, on a total of 17 patients. Procedures included root canal treatment, retreatment, vital pulp therapy and some restorative work. They also had great assistance from their colleagues at the Charles River Community Health Center, to find the patients in need.
At Boston University Department of Endodontics, three administrative staff and two dental assistants provided support and coordination for 19 residents and six faculty members to help save teeth through endodontic procedures in an exceptional event. Patients from local health centers (including Harbor Health and Dimock) and internal patients with specific financial needs were treated within the postgraduate clinic at the Goldman School of Dental Medicine. A total of 20 endodontic procedures were successfully performed on 19 patients at Boston University.
About the American Association of Endodontists: The American Association of Endodontists (AAE) is headquartered in Chicago and represents more than 8,000 members worldwide. Endodontics is one of nine dental specialties formally recognized by the American Dental Association. The AAE, founded in 1943, is dedicated to excellence in the art and science of endodontics and to the highest standard of patient care. The Association inspires its members to pursue professional advancement and personal fulfillment through education, research, advocacy, leadership, communication and service. For more information about the AAE, visit the Association’s website at aae.org. For more patient focused information, visit aae.org/patients.

A realistic environment to train future dentists
Last month, two UMKC training laboratories were equipped with 109 Intego student simulator units and two instructor units for its School of Dentistry students. The upgraded training labs now allow students to learn and practice in a realistic clinical setting from the very start as all dental procedures can be simulated under ergonomic conditions with correct instrumentation technique. The configuration of the workstations allows utmost flexibility, such as easy switching from right-handed treatments to left-handed. Thanks to its modular concept, the units are also suitable for both UMKC dental and dental hygiene curricula and include the option to add further functions and technological updates in digital dentistry in the future.
"The planning and research process were crucial to the success of this project at UMKC. Working closely with the university leaders to map their ideas and visions for the upcoming years allowed us to deliver an individually tailored product and training portfolio from start to finish,” says Peter Rössling, Dentsply Sirona Sales Director of International Special Clinic Solutions USA/Canada. “This is our first simulator installation of its kind in the US, and we look forward to bringing our technology to more students in the country.”
Universities and large clinics all around the globe from Germany to South Africa and New Zealand trust the long-term expertise of Dentsply Sirona’s ISCS division and its comprehensive approach. From consultation and planning to training and global support, ISCS ensures each customer’s unique vision is brought to life.
The high-quality equipment at UMKC has been designed and produced in Germany using particularly robust materials ideal for university settings.
UMKC – A world-class institution for excellence in oral healthcare education
Since 1881, UMKC School of Dentistry has been offering a varied and complete range of educational experiences for students of dentistry and dental hygiene, as well as students for Advanced Education and continuing education. The faculty comprises scholars, scientists and specialists—all dedicated to providing a high quality and comprehensive education, which is now enhanced by the new modern dental equipment from Dentsply Sirona. “The lab is spectacular. This will give our students more real-life experiences before performing dental care on people,” says Marsha Pyle, Dean of the UMKC School of Dentistry.
NEWPORT BEACH, Calif., November 19, 2019 - On Sept. 16, 2019, Glidewell Dental hosted California State Senator Ling Ling Chang for the grand opening and ribbon-cutting ceremony of the company’s all-new, 10,000-square-foot Education Center. True to the company’s emphasis on dental technology and research and development, the new facility features two large classrooms, state-of-the-art displays, and connectivity to numerous Glidewell campuses to promote large-scale and remote training for laboratory technicians and a range of other dental professionals who form the backbone of the company’s industry-leading dental lab services.
“I’ve toured a ton of companies and I’ve not seen anything like this,” Senator Chang said. “You have a whole environment geared toward supporting your employees, and it’s absolutely amazing.”
Imbued with CAD/CAM technology and the industry’s latest dental software and lab equipment, the Education Center advances the longstanding mission of President and CEO Jim Glidewell, CDT, to develop the careers and skillsets of the company’s employees while implementing technologies that make dentistry more precise and efficient.
“Investing our resources in R&D and our employees has always been a top priority for our company,” Glidewell said. “Our new education center embodies that principle and will help our employees as they strive toward bigger and brighter futures in dental technology.”
Senator Chang observed: “It seems like a very family-oriented environment. I was just telling Mr. Glidewell that folks constantly bring up the future of work and the concern about technology taking over, but he has been able to continue growing his workforce even with technology being part of the organization. That speaks volumes, and I’m going to bring my colleagues down here to take a tour of this amazing organization because this is truly a model.” Senator Chang went on to favorably compare Glidewell to other high-profile companies driving job growth through technological innovation.
With about 50 Glidewell managers and executives in attendance, Senator Chang commemorated the Center’s opening with an official Certificate of Recognition presented to Glidewell Dental “in celebration of your new educational facility and in honor of your commitment to the community.”
Owandy-CR2 Delivers High Quality Images with Excellent Contrast; Including Digital Radiographs Taken at Low Radiation Doses
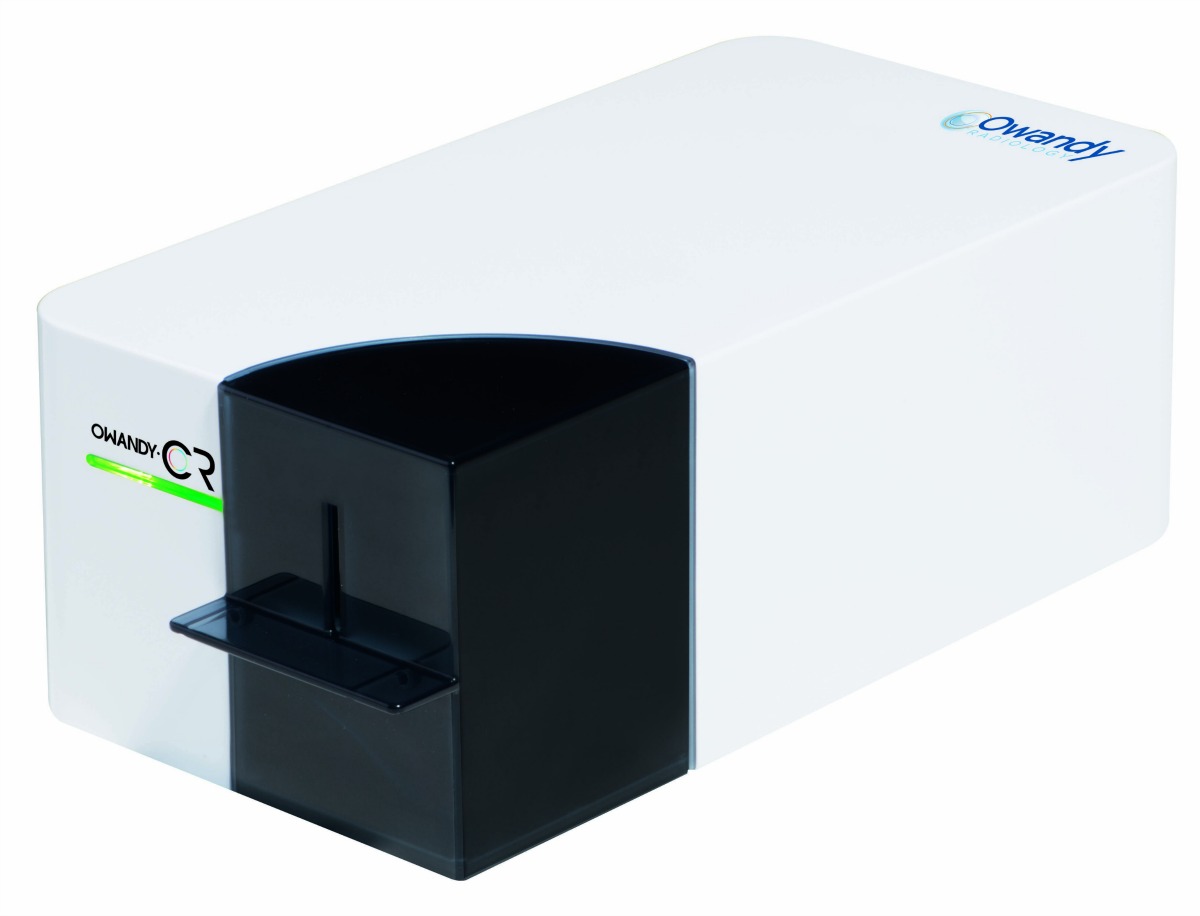
According to Owandy company spokesman Boris Loyez, “The Owandy-CR2 packs an incredible amount of performance and value into its remarkably small size. Its cutting- edge reading head produces accurate images even at low radiation doses and its specially-engineered image filters deliver high quality images with excellent contrast.”
The Owandy-CR2 is the perfect plate scanning solution for a wide range of clinical applications, including endodontics, prosthetics, implantology, periodontics, orthodontics and caries diagnosis.
This scanner is highly intuitive, thanks to sensors that automatically detect the size and insertion direction of the plates being scanned. It was designed for proprietary use with Owandy plates and can scan the following Owandy plate sizes: #0 – child, #1 – incisor block, #2 adult, #3 – bitewing, #4 – occlusal block. The complete list of Owandy-CR2 features includes the following:
· Smallest plate scanning system on the market
· No complicated user interfaces
· No buttons – Automatic plate detection and image display
· Optimized sensitivity – High definition at low doses
· Scans 4 Owandy plate sizes for optimal versatility
· Automatic wakeup and standby mode
· Ethernet or USB versions available
During the Greater New York Dental Meeting, the Owandy CR2, regularly priced at $7,190.00 will be available at the discounted price of $6.490.00 - a $700.00 savings. To download a complete list of GNYDM show specials, click here.
The launch of the CR2 is part of Owandy’s strategic plan to coordinate major product launches around its U.S. dental meeting schedule. For example, the company debuted ungraded version of its popular I-Max 3-D during the recent ADA/FDI meeting.
The launch of the CR2 is part of Owandy’s strategic plan to coordinate major product launches around its U.S. dental meeting schedule. For example, the company debuted an upgraded version of its popular I-Max 3-D Panoramic System during the recent ADA/FDI meeting. Innovative new features included improved face scanning and airway imaging, which improve diagnosis, treatment planning and patient acceptance for the most common and profitable dental procedures, including dental sleep medicine. The I-Max 3-D will also be on display at GNYDM.
For more information about the Owandy-CR2 intraoral imaging plate scanner and other Owandy innovations, visit www.owandy.com, call 203-745-0575 or e-mail at sales@owandyus.com. Distributor inquiries are always welcome.
About Owandy Radiology, Inc.
Headquartered in France, and serving North America from Mount Laurel, New Jersey, Owandy Radiology is a global leader in the manufacture of dental radiology hardware and imaging software. Its products are distributed through dental dealers across the USA, and in 50 countries world-wide, on every continent.

In the B2B, Healthcare / Medical / Nursing category, Larry Cohen, Chairman and Chief Customer Advocate for Benco Dental received an Eddie for “Larry’s Collection,” a column on dental artifacts culled from hundreds in his personal reserve. Kristie Ceruti, the magazine’s Digital Editor, garnered an Eddie for a series of articles that takes an architectural and interior design tour of the country’s most impressive dental practices. IncisalEdgeMagazine.com received an Eddie for website with a revamp of the digital product by Christopher Cruz, Kevin Garubba, Kristie Ceruti, Rachel Pugh and Loriah Webby.
Categories in which Incisal Edge received honorable mention awards include:
Eddies, awards for journalism
· Video, B2B, Healthcare / Medical / Nursing: Incisal Edge, “I Am Lucy Hobbs” by Eric Larsen
Ozzies, awards for design
· Cover Design, B2B, Above 100,000 Circulation: Incisal Edge, Fall 2018 by Loriah Webby
· Feature Design, B2B, Above 100,000 Circulation: Incisal Edge, 40 Under 40: the 8th annual Kind of Blue, Fall 2018 by Loriah Webby
· Overall Design (Single Magazine Issue), B2B: Incisal Edge, True North: Winter 2019 by the Incisal Edge Design team of Sharon Fiorini, Donna Shrader, Jimmy Musto and Loriah Webby.
· Photography, B2B, Print: Incisal Edge, True North: Winter 2019 by Eric Larsen
· Site Design, B2B: Incisal Edge, IncisalEdgeMagazine.com by Christopher Cruz and Kevin Garubba

The Folio: team, along with their panel of 200+ judges, received more than 2,000 entries submitted this year in 33 categories. Other publishers among the 2019 finalists are: ESPN the Magazine, Forbes Media, Hearst Magazines, Meredith Corporation and Variety. View the full list here.
Incisal Edge is a previous recipient of an Ozzie Award for Under40Summit.com in the category of Site Design for in 2018. To learn more about Incisal Edge, visit: https://www.incisaledgemagazine.com/
CAPTIONS:
1. 18_IncisalEdge_Larry’s Collection: — Incisal Edge earns top spot in three categories and honorable mention in nine during the 2019 FOLIO: Eddie & Ozzie Awards that celebrate excellence in journalism and design across all sectors of publishing. In the B2B, Healthcare / Medical / Nursing category, Larry Cohen, Chairman and Chief Customer Advocate for Benco Dental received an Eddie for “Larry’s Collection,” (shown) a column on dental artifacts culled from hundreds in his personal reserve.
2. 18_IncisalEdge_SeriesofArticles_Ceruti.jpg: — Incisal Edge earns top spot in three categories and honorable mention in nine during the 2019 FOLIO: Eddie & Ozzie Awards that celebrate excellence in journalism and design across all sectors of publishing. Kristie Ceruti (shown) garnered an Eddie for a series of articles that takes an architectural and interior design tour of the country’s most impressive dental practices.
About Incisal Edge
Knowledge. Success. Life. Those three words, printed on the cover of every issue, guide editorial content for the national’s premier dental lifestyle magazine -- Incisal Edge. Published by Benco Dental since 1997, Incisal Edge celebrates dentists’ achievements both inside the operatory and during their hard-earned downtime. Known for its “40 Under 40 — America’s Best Young Dentists,” which highlights the country’s brightest rising stars, the magazine’s content ranges from “The 32 Most Influential People in Dentistry” and news about the latest techniques, tools, and dental practice designs, to the best in travel, automobiles, and timepieces. Incisal Edge magazine’s print edition reaches 130,000 readers quarterly via a direct mail distribution package with Dentaltown magazine. Digitally, incisaledgemagazine.com, leads with dynamic visuals and follows with exclusive web content, an improved mobile experience, and a centralized nomination hub for the magazine’s signature awards: the aforementioned 40 Under 40, the Lucy Hobbs Project Awards for Exemplary Women in Dentistry and the Incisal Edge Design Competition for interior design, architecture and space planning within the dental profession.
About Benco Dental
Benco Dental, headquartered in Northeastern Pennsylvania, is the largest privately-owned dental distributor in the United States, offering a full array of supplies, equipment and services to dentists across the nation. Founded in 1930 by Benjamin Cohen, the company has remained focused on its unique mission to drive dentistry forward through innovative solutions and caring family culture. Within the past 88 years, the company has grown from a single downtown location to a national network that includes five distribution centers, and three design showrooms, one at the company’s Pa. headquarters, one in Southern California, and one in Texas. Benco, which has been named one of Fortune Best Workplaces in Health Care and Biopharma for the past three years, one of the NAFE Top Companies for Executive Women for the second consecutive year, and Pennsylvania’s Best Places to Work® for 12 of the past 14 years, is proud to feature a highly skilled team of more than 400 professionally trained sales representatives and over 300 factory-trained service technicians. For more information, visit benco.com or call 1.800.GO.BENCO.

“Convergent Dental is a dynamic company comprised of a highly dedicated team of people,” said Mr. Craig. “After meeting Michael Cataldo, I knew the partnership would be a perfect fit. Michael is a high-integrity guy with a lot of energy, who thinks differently.” He continued, “But beyond the people and culture is Solea. I instantly loved the technology, and remember thinking, ‘this laser is going to reinvent dentistry.’ And I want to be on the forefront of that revolution.”
According to Mr. Craig’s personal philosophy, the foundation of successful sales is the combination of a product that helps customers achieve their goals and sales people who guide them to great conclusions through active listening. “Solea’s ability to enrich patient experiences, improve office efficiencies, enhance clinical outcomes, and increase new patient flow is absolutely unique,” remarked the industry veteran. “By seeking first to understand each dentist’s individual ambitions, the sales team can help them leverage Solea’s many unique benefits to meet their goals.”
“Solea is a progressive technology that demands a forward-thinking sales approach implemented by a skilled and ethical sales team,” said Michael Cataldo, CEO of Convergent Dental. “We are proud of what the team has accomplished so far and know the addition of Wayne as National VP of Sales will provide the leadership needed to take Solea and Convergent Dental to the next level.”
For more information about Solea, visit convergentdental.com or call 844.GOSOLEA.
About Convergent Dental, Inc.
Convergent Dental, Inc. is a privately owned dental equipment and technology company. Solea® is the only computer-aided, CO2 laser system to be cleared by the FDA for all-tissue indications. With Solea’s unique wavelength and computer controls, dentists can reliably perform procedures anesthesia-free, blood-free, suture-free, and pain-free. Reliably anesthesia-free and blood-free procedures pay huge dividends for patients in the form of a vastly improved dental experience and dentists in terms of significant practice growth. For more information, please visit www.convergentdental.com. Follow the company on Twitter, Facebook, LinkedIn and Instagram.
Until now, toothpaste tubes haven't been recyclable because most are made of a mixed material that doesn't have a second life and has to be landfilled. The #2 plastic continues to have a strong recycling stream and is the same material used in most laundry detergent bottles. The new Tom's of Maine recyclable tube is designed to be "circular," so that the material can be re-processed into new products and packaging.
"We're thrilled to offer a first-of-its-kind recyclable toothpaste tube that's been recognized by the Association of Plastic Recyclers, which sets the standard for North America. There is no oral care or personal care tube on the market with this APR recognition," said Esi Seng, general manager at Tom's of Maine. "We're already hard at work engaging with The Recycling Partnership and their network to communicate with recycling centers and win their acceptance of our recyclable tube. We're proud to be blazing a trail for other toothpaste brands to follow," Seng added.
Tom's of Maine Antiplaque & Whitening Peppermint Natural Toothpaste will be the first variant in the new tube, available on shelves in the coming weeks, with all full size Tom's of Maine toothpastes in the new recyclable tube by the end of 2020. For more information, please visit: https://www.tomsofmaine.com/our-promise/caring-for-the-planet.
"When it comes to recycling, shoppers interested in natural products are also more committed, active participants in working to keep waste out of landfills," said Julie Sprague, stewardship manager at Tom's of Maine. "This is another commitment we're making as a company guided by a rigorous set of standards called our Stewardship Model, which ensures we're operating sustainably and responsibly every day. Taking care of the planet is a goal we all share and this exciting launch is a new way we can work together in this ongoing effort," Sprague added.
To recycle the tube at home, people should check the back of their tube for the blue "flag" that tells you what to do: once empty, replace cap and recycle with #2 plastics. Tom's of Maine tubes without the blue flag haven't yet transitioned to the new recyclable material. Recycling practices vary by municipality and if a town doesn't accept #2 plastic, the Tom's of Maine Natural Care Recycling Program, a partnership with TerraCycle, is a recommended option for recycling all personal and oral care packaging regardless of the brand.
Tom's of Maine recently became a Certified B Corporation®, joining the ranks of top socially responsible companies. Through this accreditation, the company continues its commitment to transparency, caring for the planet and communities, and setting a positive example for future generations. 2020 marks the 50th anniversary of the brand still headquartered in Kennebunk, Maine. You can learn more about Tom's of Maine, its complete product portfolio and how the company makes its products by visiting www.TomsofMaine.com and www.Facebook.com/TomsofMaine.
Tom's of Maine has been making safe, effective natural personal care products for 49 years. It all began when Tom and Kate Chappell moved to Maine in 1968 looking for a healthier, simpler life for their growing family. And when they couldn't find personal care products that were free from artificial flavors, fragrances, sweeteners, colors and preservatives, they decided to make their own. Tom's of Maine products – including toothpaste, deodorant, mouthwash, antiperspirant, bar soap, body wash, dental floss, and toothbrushes – are made from naturally sourced and naturally derived ingredients and never tested on animals. A Certified B Corporation, Tom's of Maine is committed to upholding a purpose-driven business and has a long-standing commitment to supporting nature and healthy families. Tom's of Maine has supported hundreds of nonprofits by giving back 10% of its profits, and employees are encouraged to use 5% of their paid time (12 days) volunteering for causes they are passionate about. Most Tom's of Maine products are vegan, kosher, halal-certified and gluten-free. All packaging is recyclable through a partnership with upcycling leader TerraCycle or participating municipalities. Visit us online at https://www.tomsofmaine.com/ or at https://www.facebook.com/TomsofMaine.
Keystone Industries® has obtained 510k clearance by the US FDA to sell its revolutionary 3D printing night guard and splint resin in the United States, joining Canada and the EU as territories where KeySplint Soft can be purchased. KeySplint Soft is unlike any other 3D printing material. Without being brittle, it is tough and durable like a hard splint to withstand the forces of bruxism, yet flexible for patient comfort. It is abrasion and fracture resistant. KeySplint Soft is made in the USA, has 3 years of guaranteed shelf life, and is a highly efficient and profitable way to make splints and night guards.
Keystone Industries has two shades available—an aesthetic clear version that is optimized exclusively for the Carbon® Digital Manufacturing Platform as well as an open-source version with a slight violet tint. The former is available exclusively through Zahn Dental.
Keystone’s patent-pending formulation provides a unique solution for dental labs and clinicians: a 3D printed night guard and splint that is flexible and comfortable, yet tough and durable, without brittleness.
“We worked for years to create a unique, safe, and effective night guard solution for 3D printing,” said Ira Rosenau, President of Keystone’s dental group. “With the added production efficiency of 3D printing, KeySplint Soft offers a top quality and unique splint solution that creates a significant value for the laboratory and a terrific protective device for the patient.”
Concerning the 510k clearance for KeySplint Soft, Mr. Rosenau noted, “This is a significant event in that the FDA has not reviewed and cleared many 3D printing materials as medical devices, and KeySplint Soft is believed to be just the second FDA clearance for splint and night guard applications. In order to compliantly 3D print night guards or splints in the United States, users need to look for materials that have undergone and cleared the FDA 510k process. We are pleased to be one of the first compliant splint solutions here in the USA.”
Keystone’s exclusive arrangement with Carbon on a clear version of KeySplint Soft is particularly noteworthy for users of the Carbon Digital Manufacturing Platform seeking high-quality, high-speed splint production.
“We’re excited that with the FDA clearance, we can now make the KeySplint Soft Clear material available to Carbon’s global network of dental partners and customers via the Carbon Digital Manufacturing Platform,” said Brian Ganey, Vice President of Carbon’s Oral Health Business. “We believe the KeySplint Soft™ Clear resin is the most esthetic, best-in-class material for night guards and bite splint applications.”
The combination of the unique KeySplint Soft Clear resin and Carbon’s robust M series printers allows labs to produce 6x more splints than traditional production methods while increasing profit per unit by nearly 75%.
Early users have praised KeySplint Soft as a 3D solution the market has been seeking. Ashley Byrne, an experienced dental laboratory owner from Oxford, England, has been using KeySplint Soft Clear in his Carbon printers.
Byrne notes, “I’ve never used a material like it. I'm just blown away by how good it is. It is soft but super strong, incredibly comfortable to wear and printed on our Carbon M2 printers. Many splint materials are simply too brittle and break easily. However, this material is a game changer in the world of occlusal splints and keeps blowing our mind.”
Jay Patrick has spent years focusing on night guard and splint manufacturing, and now uses the Asiga Max (UV 385) for his 3D printing needs. After using KeySplint Soft, Jay remarked, “I honestly believe this will change printed splints forever. It has unbelievable fracture-resistance and mind-blowing elastic memory.”
Keystone Industries has validated KeySplint Soft in several printers and with several post-cure units, including printers from Carbon, Asiga, MiiCraft, and others, with additional validations ongoing regularly. Keystone updates its validations at https://keyprint.keystoneindustries.com/printer-validation/.
Keystone has been a trusted dental manufacturer since 1908 and has developed an expertise over several decades in manufacturing photopolymer formulations. The KeyPrint® dental 3D printing solutions are manufactured in the USA and stocked in Keystone’s US and EU fulfilment centers.
Using its expertise in manufacturing both dental acrylics (like Diamond D® high-impact denture base) and night guards and splints (such as the industry-leading Pro-Form® line of thermoplastics), Keystone Industries has seen splint fabrication evolve from the tedious hand-crafting of cured acrylic splints to thermoforming laminate sheets over hand-poured stone models. With the digital dental revolution, 3D printing KeySplint Soft yields a high quality, accurate and safe device at significantly reduced labor and material costs compared to other methods. With digital storage available, a lost or damaged splint can be re-printed easily, without any additional rework or patient visits.
For more information about KeySplint Soft and the entire KeyPrint line of 3D printing resins, visit https://keyprint.keystoneindustries.com/.
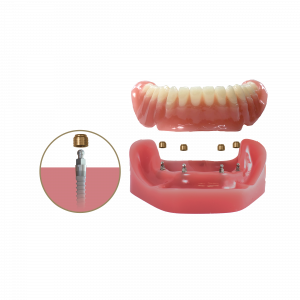
Some of what attendees can expect to learn are basic and advanced denture stabilization case treatments, a demonstration of hands-on surgical prosthetic procedures, treatment indications and contraindications, and valuable practice building strategies. Also introduced is the new “Treatment Plan Workshop." This workshop enables attendees to successfully turn their challenging cases involving limitations on bone, time, or finances into accepted treatments.
2020 Mini Implant course dates and locations:
January 25 – Atlanta, GA
February 8 - New Orleans LA
March 20 – Tampa Bay, FL
April 17 – Philadelphia, PA
May 9 – Chicago, IL
May 16 – St. Louis, MO
October 24- Oklahoma City, OK
November 14- Weehawken, NJ
December 12- Atlanta, GA
All courses slated between January to May are now open for enrollment. To register or learn more, visit sterngold.com/courses or call 800-243-9942.