 GlaxoSmithKline (LSE/NYSE: GSK) today announced a donation of $1 million to Direct Relief on behalf of consumers who have purchased its consumer healthcare products since the onset of the COVID-19 pandemic. According to the COVID Tracking Project, hospitalizations have more than doubled in less than two months. GSK recognizes that with cases rising so rapidly, there is a critical need to continuously support health care workers. The monetary donation will be used to purchase personal protective equipment (PPE) and essential medical items for U.S. health workers on the front line dealing with this unrelenting crisis.
GlaxoSmithKline (LSE/NYSE: GSK) today announced a donation of $1 million to Direct Relief on behalf of consumers who have purchased its consumer healthcare products since the onset of the COVID-19 pandemic. According to the COVID Tracking Project, hospitalizations have more than doubled in less than two months. GSK recognizes that with cases rising so rapidly, there is a critical need to continuously support health care workers. The monetary donation will be used to purchase personal protective equipment (PPE) and essential medical items for U.S. health workers on the front line dealing with this unrelenting crisis.
Since the outbreak, GSK has been providing its scientific expertise to support the global response to COVID-19 and ensuring its global supply chain continues to deliver vital medicines and consumer healthcare products to the people who depend on them. GSK Consumer Healthcare's portfolio includes leading brands such as Sensodyne, Advil, Voltaren, Excedrin, Theraflu, Flonase, Centrum, and Emergen-C.
"We are so proud to partner with Direct Relief to support the amazing work they are doing to ensure health professionals have the essential items that they need as the COVID-19 pandemic continues," said Lisa Paley General Manager, U.S. and Puerto Rico for GSK Consumer Healthcare. "We know how important maintaining health is, especially as newly diagnosed coronavirus cases and hospitalizations continue to rise. As one of the leading consumer healthcare companies, we have an obligation to pay it forward and give back to frontline workers who have been working tirelessly to keep us safe over the past nine months. Our continued focus remains, ensuring healthcare worker's needs are being met, staying vigilant as the pandemic continues and keeping a steadfast focus on how critical these needs really are."
Direct Relief is coordinating with public health authorities, nonprofit organizations, and businesses to provide personal protective equipment and essential medical items to health workers responding to COVID-19. Since January, Direct Relief has delivered more than 46 million N95 and surgical masks, more than 8 million gloves, and tens of thousands of protective suits and other items to help safeguard health workers
"Direct Relief is so deeply grateful for the leadership and commitment reflected by GSK's action today, which is both keenly needed and will be put to immediate use," said Thomas Tighe, CEO and President of Direct Relief. "This is a perfect example of what's needed as we all face this historic threat to the health of people everywhere."
Since the outbreak, GSK has been providing its scientific expertise to support the global response to COVID-19 and ensuring its global supply chain continues to deliver vital medicines and consumer healthcare products to the people who depend on them.
GSK Consumer Healthcare has been supporting U.S. communities and its employees nationwide in the wake of the COVID-19 crisis in many ways, including:
Participated in a major industry-wide movement to amplify one resonant message: #StayHome. Save lives. GSK Consumer Healthcare brands in the U.S. added a roof icon to their brand logo and shared assets on brand social channels with the hashtags #StayHome and #AloneTogether.
Coordinated a donation of ChapStick and Abreva to 12 high-impact areas, including New York, Michigan, California, Washington, Florida, Texas, Illinois, Ohio and Maryland.
Sponsored the Global Citizen special, One World: Together at Home.
Donated $10,000 to the NJ Community Food Bank, which serves several hard-hit New Jersey areas. In March, the organization supplied enough food for 4.8 million meals to those in need.
Shipped 3,000 care packages to every essential GSK Consumer Healthcare manufacturing and distribution center employee across the country.
GSK Consumer Healthcare is also encouraging customers to #BeWellStayWell by taking care of themselves and their families with health and wellness products, more information can be found at BeWellandStayWell.com
Globally, GSK is closely monitoring the COVID-19 pandemic and supporting efforts to tackle the virus. The company is collaborating with companies and research groups across the world working on promising COVID-19 vaccine candidates through the use of its innovative vaccine adjuvant technology. It is also supporting screening and research into potential medicines for COVID-19, as well as providing expertise and financial support to relief organizations. For more information on GSK's global efforts, visit https://www.gsk.com/en-gb/media/resource-centre/our-contribution-to-the-fight-against-2019-ncov/.
About Direct Relief
A humanitarian organization committed to improving the health and lives of people affected by poverty or emergencies, Direct Relief delivers lifesaving medical resources throughout the world to communities in need—without regard to politics, religion, or ability to pay. For more information, please visit https://www.DirectRelief.org.
About GSK Consumer Healthcare
GSK Consumer Healthcare combines science and consumer insights to create innovative world-class health care brands that consumers trust, and experts recommend for oral health, pain relief, respiratory and wellness.
About GSK
GSK is a science-led global healthcare company with a special purpose: to help people do more, feel better, live longer. For further information please visit gsk.com.




 GlaxoSmithKline (LSE/NYSE: GSK) today announced a donation of $1 million to Direct Relief on behalf of consumers who have purchased its consumer healthcare products since the onset of the COVID-19 pandemic. According to the COVID Tracking Project, hospitalizations have more than doubled in less than two months. GSK recognizes that with cases rising so rapidly, there is a critical need to continuously support health care workers. The monetary donation will be used to purchase personal protective equipment (PPE) and essential medical items for U.S. health workers on the front line dealing with this unrelenting crisis.
GlaxoSmithKline (LSE/NYSE: GSK) today announced a donation of $1 million to Direct Relief on behalf of consumers who have purchased its consumer healthcare products since the onset of the COVID-19 pandemic. According to the COVID Tracking Project, hospitalizations have more than doubled in less than two months. GSK recognizes that with cases rising so rapidly, there is a critical need to continuously support health care workers. The monetary donation will be used to purchase personal protective equipment (PPE) and essential medical items for U.S. health workers on the front line dealing with this unrelenting crisis.
 Today, hum by Colgate announced its partnership with leading meditation and mindfulness app, Headspace, to help consumers stress less and smile more by creating healthier daily habits. The partnership encourages consumers to treat the existing routines in their day - whether it's going for a walk outside or brushing their teeth - as a chance to observe and reflect on their thoughts.
Today, hum by Colgate announced its partnership with leading meditation and mindfulness app, Headspace, to help consumers stress less and smile more by creating healthier daily habits. The partnership encourages consumers to treat the existing routines in their day - whether it's going for a walk outside or brushing their teeth - as a chance to observe and reflect on their thoughts.
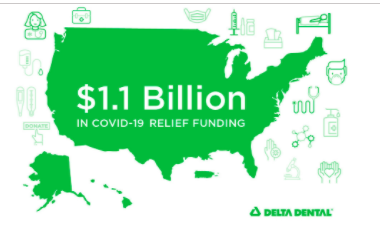 Today, Delta Dental announced its COVID-19 relief efforts are expected to reach $1.1 billion by early 2021. This significant financial commitment builds on Delta Dental's 65-year history of supporting America's oral health and continues its leadership in the provision of support to the U.S. dental marketplace during the COVID-19 pandemic.
Today, Delta Dental announced its COVID-19 relief efforts are expected to reach $1.1 billion by early 2021. This significant financial commitment builds on Delta Dental's 65-year history of supporting America's oral health and continues its leadership in the provision of support to the U.S. dental marketplace during the COVID-19 pandemic.
 Stratasys Ltd. has announced it signed an agreement to acquire 3D printing start-up Origin Inc. in a transaction for total consideration of up to $100 million, including cash and stock. The merger enables Stratasys to expand its leadership through innovation in the fast-growing mass production parts segment with a next-generation photopolymer platform. Subject to various approvals and other closing conditions, the acquisition is expected to close in January 2021.
Stratasys Ltd. has announced it signed an agreement to acquire 3D printing start-up Origin Inc. in a transaction for total consideration of up to $100 million, including cash and stock. The merger enables Stratasys to expand its leadership through innovation in the fast-growing mass production parts segment with a next-generation photopolymer platform. Subject to various approvals and other closing conditions, the acquisition is expected to close in January 2021. Sonendo, Inc., a leading dental technology company and developer of the GentleWave® System, today announced that more than 500,000 patients have been treated with the GentleWave Procedure.
Sonendo, Inc., a leading dental technology company and developer of the GentleWave® System, today announced that more than 500,000 patients have been treated with the GentleWave Procedure. Formlabs, a leading 3D printing company, today announced the expansion of its dental material offerings with the launch of Permanent Crown Resin and Soft Tissue Starter Pack. The introduction of these new materials empowers dental offices and labs to further digitize restorative dentistry and offer patients 3D printed long-term restorations such as fillings, crowns (“caps”), bridges, and implants. 3D printing permanent crowns is a completely new application to the dental industry, and with Formlabs’ new Permanent Crown Resin, the company is one of the first 3D printing manufacturers to offer a high quality solution with a low upfront equipment cost.
Formlabs, a leading 3D printing company, today announced the expansion of its dental material offerings with the launch of Permanent Crown Resin and Soft Tissue Starter Pack. The introduction of these new materials empowers dental offices and labs to further digitize restorative dentistry and offer patients 3D printed long-term restorations such as fillings, crowns (“caps”), bridges, and implants. 3D printing permanent crowns is a completely new application to the dental industry, and with Formlabs’ new Permanent Crown Resin, the company is one of the first 3D printing manufacturers to offer a high quality solution with a low upfront equipment cost. Leading innovator of endodontics technology, Kerr Dental, announces the launch of the ZenFlexTMNiTi Rotary Shaping File, featuring high cutting efficiency, an ideal balance of strength and flexibility, and a minimally invasive design. Initial comparative studies against other leading files on the market indicate that ZenFlex delivers exceptional resistance to torsional stress and cyclic fatigue, while offering the flexibility and controlled memory to accommodate even the most complex canal anatomies.
Leading innovator of endodontics technology, Kerr Dental, announces the launch of the ZenFlexTMNiTi Rotary Shaping File, featuring high cutting efficiency, an ideal balance of strength and flexibility, and a minimally invasive design. Initial comparative studies against other leading files on the market indicate that ZenFlex delivers exceptional resistance to torsional stress and cyclic fatigue, while offering the flexibility and controlled memory to accommodate even the most complex canal anatomies.
