Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global leader in musculoskeletal healthcare, today announced a multinational distribution agreement with Align Technology, Inc. (NASDAQ: ALGN), for the award-winning iTero Element family of intraoral scanners. Terms of the agreement have not been disclosed. The agreement expands Zimmer Biomet's global footprint in the rapidly growing market for digital restorative dentistry solutions.
"Zimmer Biomet is committed to offering best-in-class solutions and technology tailored to dental professionals' needs, and this partnership with Align Technology to complement our portfolio of fully digital solutions is another important step in that direction," said Pedro Malha, President, Zimmer Biomet Dental.
Together, the iTero Element scanner and Zimmer Biomet's dental solutions will deliver multiple digital workflows for dental professionals and laboratories, enabling efficient business-to-business collaboration and offering patients an optimal treatment experience.
"We are excited to be partnering with Zimmer Biomet to demonstrate our companies' commitment to supporting dental professionals on their journey to digital dentistry," said Yuval Shaked, Senior Vice President and Managing Director, iTero Scanner and Services. "A key differentiator in the evolution to a digital practice and dental ecosystem is clinical education. Through this partnership, the iTero scanner becomes the preferred intraoral scanner used in the U.S. and European Zimmer Biomet Institutes, which train thousands of dental professionals annually in an interactive learning environment with the ultimate goal of improved clinical outcomes."
iTero Element scanners and services are now available through Zimmer Biomet Dental in Europe, with distribution expanding to the U.S., Canada and Japan starting in October 2019.
About Zimmer Biomet
Founded in 1927 and headquartered in Warsaw, Indiana, Zimmer Biomet is a global leader in musculoskeletal healthcare. We design, manufacture and market orthopedic reconstructive products; sports medicine, biologics, extremities and trauma products; office-based technologies; spine, craniomaxillofacial and thoracic products; dental implants; and related surgical products.
We collaborate with healthcare professionals around the globe to advance the pace of innovation. Our products and solutions help treat patients suffering from disorders of, or injuries to, bones, joints or supporting soft tissues. Together with healthcare professionals, we help millions of people live better lives.
We have operations in more than 25 countries around the world and sell products in more than 100 countries. For more information, visit www.zimmerbiomet.com, or follow Zimmer Biomet on Twitter at www.twitter.com/zimmerbiomet.
About Align Technology, Inc.
Align Technology designs and manufactures the Invisalign® system, the most advanced clear aligner system in the world, and iTero® intraoral scanners and services. Align's products help dental professionals achieve the clinical results they expect and deliver effective, cutting-edge dental options to their patients. Visit www.aligntech.com for more information.
For additional information about the Invisalign system, or to find an Invisalign doctor in your area, please visit www.invisalign.com. For additional information about iTero digital scanning system, please visit www.itero.com.
Cautionary Statement Regarding Forward-Looking Statements
This release contains forward-looking statements within the meaning of federal securities laws, including, among others, statements concerning Zimmer Biomet's expectations, plans, prospects, and product and service offerings. Such statements are based upon the current beliefs, expectations and assumptions of management and are subject to significant risks, uncertainties and changes in circumstances that could cause actual outcomes and results to differ materially from the forward-looking statements. For a list and description of some of such risks and uncertainties, see Zimmer Biomet's periodic reports filed with the U.S. Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended December 31, 2018. These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in Zimmer Biomet's filings with the SEC. Forward-looking statements speak only as of the date they are made, and Zimmer Biomet expressly disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Readers of this release are cautioned not to rely on these forward-looking statements, since there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this release.






 Gary Hubbard, DDS, FAACD, has just been named the 85th Accredited Fellow of the American Academy of Cosmetic Dentistry (AACD). The purpose of Fellowship is to recognize the highest level of achievement for members in accordance with the AACD's mission of education and excellence.
Gary Hubbard, DDS, FAACD, has just been named the 85th Accredited Fellow of the American Academy of Cosmetic Dentistry (AACD). The purpose of Fellowship is to recognize the highest level of achievement for members in accordance with the AACD's mission of education and excellence.
 Dante Labs announced today the 10,000 Oral Microbiome Project, aimed at improving the oral care of individuals by leveraging the latest DNA sequencing technologies. The project will improve the understanding of the relationship between diseases and the DNA of the microbes in our mouths - including gingivitis and gum diseases.
Dante Labs announced today the 10,000 Oral Microbiome Project, aimed at improving the oral care of individuals by leveraging the latest DNA sequencing technologies. The project will improve the understanding of the relationship between diseases and the DNA of the microbes in our mouths - including gingivitis and gum diseases.


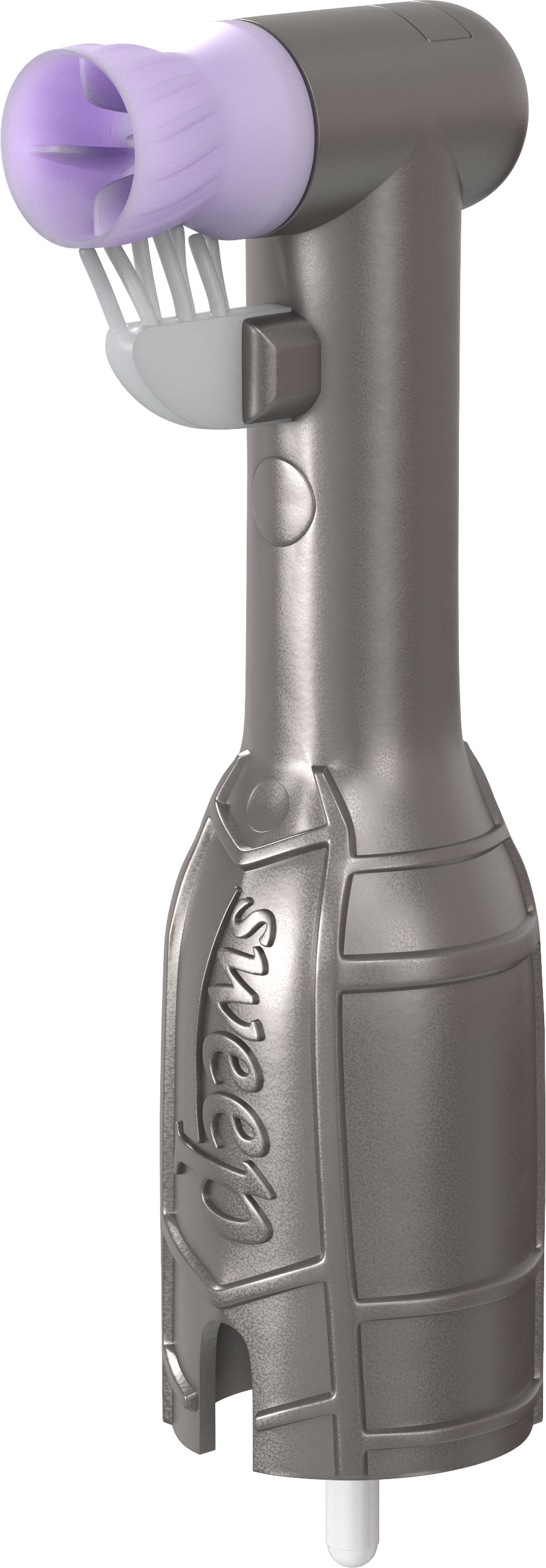 Ultradent Products, Inc., a leading developer and manufacturer of high-tech dental materials, is unveiling the latest innovation in disposable prophy angles (DPAs)—the Ultrapro Tx Sweep DPA.
Ultradent Products, Inc., a leading developer and manufacturer of high-tech dental materials, is unveiling the latest innovation in disposable prophy angles (DPAs)—the Ultrapro Tx Sweep DPA.
