Glidewell Dental today announced an agreement with Align Technology, Inc. to distribute the iTero Element®intraoral scanning system in North America with the newly unveiled glidewell.io™ In-Office Solution, a chairside restorative ecosystem set to simplify the process of prescribing and delivering laboratory-quality dental restorations.
The glidewell.io Solution will provide a streamlined workflow with Align’s iTero Element scanner and Glidewell’s fastdesign.io™ Software, which serves to auto-design restorations for the clinician’s approval or communicate with the lab’s digital design experts as needed. Final designs can be used to prescribe a laboratory restoration or sent to the fastmill.io™ In-Office Unit for immediate chairside milling.
“The promise of convenience offered by chairside milling systems tends to be offset by the cost and complexity of adoption,” said Stephenie Goddard, vice president of business operations at Glidewell Dental. “In contrast, glidewell.io uses artificial intelligence and other advanced technologies to effectively streamline the restorative process, enabling clinicians to focus on the needs and comfort of their patients.”
Touting the iTero Element scanner’s technical capabilities, Mike Selberis, Glidewell Dental’s chief technology officer, said: “An accurate restoration is almost entirely contingent upon an accurate scan. Beyond its impressive speed and inimitable precision, the intuitive interface and integrated training resources of the iTero Element scanner make it an easy choice as our preferred entry point for clinicians looking to tap into the full range of services provided by glidewell.io.”
“This agreement represents a win-win for dentists and their patients,” said Raphael Pascaud, Align Technology chief marketing, portfolio & business development officer and vice president iTero scanner and services. “The convenience and precision of intraoral scanning continues to replace conventional impression-taking techniques and enables an efficient digital start to the restorative treatment process. The natural extension of Glidewell’s digital production processes into a chairside form factor is a key driver for same-day dentistry and fits well with our strategy to transform the industry through digital products and solutions.”
“Align has long been at the forefront of digital dental technology,” said Jim Glidewell, founder and president of Glidewell Dental. “Their pioneering spirit and drive for continued innovation are very much in line with our company’s goal to remove barriers to patient treatment, and I couldn’t be more excited about this dynamic pairing.”
The glidewell.io In-Office Solution featuring the iTero Element scanner is being introduced and previewed to a select group of clinicians attending the inaugural Glidewell Dental Symposium today in Dallas, Texas. The next hands-on opportunity will be for attendees of the 2017 Greater New York Dental Meeting, taking place at the Jacob K. Javits Convention Center Nov. 24–29. The glidewell.io In-Office Solution featuring the iTero Element scanner is expected to be available to North American customers in Q1 of 2018. Glidewell customers can contact their Glidewell sales representative to place an order beginning Nov. 17.






 In conjunction with the European Federation of Periodontology (EFP), the American Academy of Periodontology (AAP) hosted the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions Nov. 9 -11 at the Gleacher Center in Chicago. More than 100 international researchers, educators, and clinicians gathered to review the latest literature and come to consensus on up-to-date guidelines for periodontal and peri-implant disease diagnosis and definition.
In conjunction with the European Federation of Periodontology (EFP), the American Academy of Periodontology (AAP) hosted the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions Nov. 9 -11 at the Gleacher Center in Chicago. More than 100 international researchers, educators, and clinicians gathered to review the latest literature and come to consensus on up-to-date guidelines for periodontal and peri-implant disease diagnosis and definition.
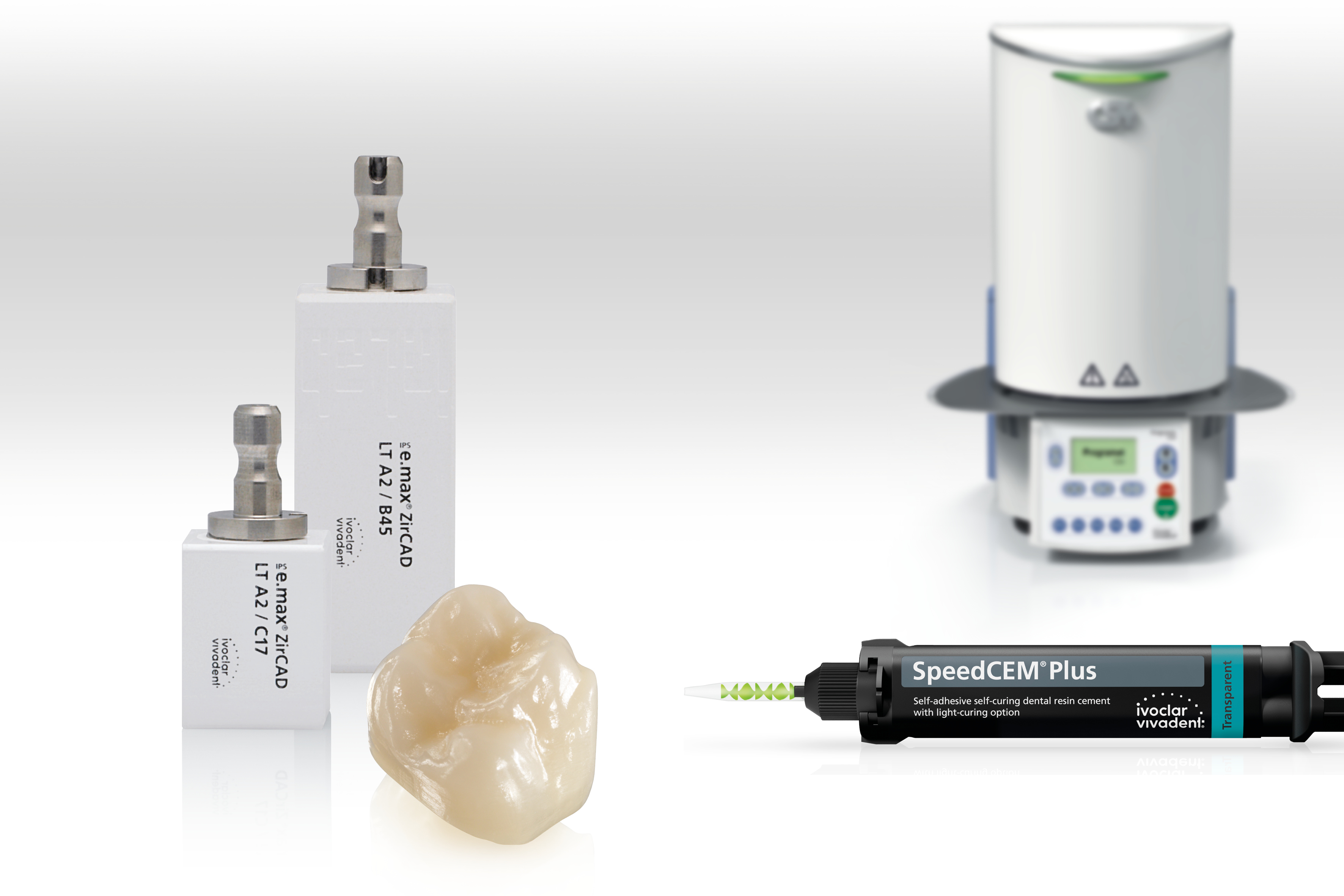 LT blocks of the IPS e.max ZirCAD for Cerec range are now available in four additional shades.
LT blocks of the IPS e.max ZirCAD for Cerec range are now available in four additional shades. Pioneer Lasers, LLC, is pleased to announce the return of two top-selling diode lasers along with an epic Q4 promotion to the dental community. Pioneer Lasers have been serving the dental industry since 2003 and over 10,000 units sold under the venerated Odyssey 2.4G brands through its distributor. They are now renamed the Pioneer Elite and Pioneer Pro models.
Pioneer Lasers, LLC, is pleased to announce the return of two top-selling diode lasers along with an epic Q4 promotion to the dental community. Pioneer Lasers have been serving the dental industry since 2003 and over 10,000 units sold under the venerated Odyssey 2.4G brands through its distributor. They are now renamed the Pioneer Elite and Pioneer Pro models.
