Preventing Implant Failure Before Placement
The technician plays a valuable role in achieving successful outcomes
Many factors affect the success or failure of dental implant restoration. They include patient characteristics (eg, smoking status, presence of diabetes mellitus), surgical technique (eg, experience, training, skill level), restoration quality (eg, type, ability to clean and maintain), and the maintenance and follow-up care.1
Often, the technician determines the restoration type and design. This is arguably the most challenging component related to implant success, particularly when the restoration must compensate for site, depth, or angulation deficiencies that result from the implant placement. Usually, these can be addressed, but this resolution will always involve compromise at the restoration level.
A common solution is to use a cemented implant restoration. Developed to mimic the tooth-cemented restoration, it has helped overcome implant-positioning challenges.2 However, issues with the cementation process for dental implants are now being observed in the literature.3-5
Residual excess cement around the implant has been linked with peri-implant disease,6 leading the American Academy of Periodontics in 2013 to list this as a major risk factor in implant failure. Several academies, including the American Academy of Restorative Dentistry,7 have also highlighted this as being a significant clinical concern.
The Role of the Technician
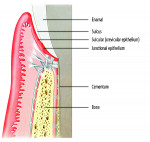
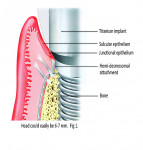
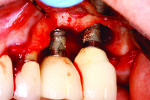
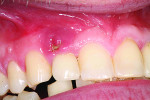
Firstly, understand that the requirements of the implant restoration are completely different from the natural tooth. With a natural tooth, the attachment of the soft tissues produce a tight, distinct seal through cementum on the tooth root and mineralized Sharpey’s fiber insertion. For an implant, the soft tissues adhere to an implant surface via a fragile hemidesmosomal attachment—the weakest cell attachment known in the human body. The implant attachment can be readily disrupted by cement flow being pushed into the tissues as the crown seats8 (Figure 1 and Figure 2).
Secondly, consider the cement margin depth. It has been suggested to place the cemented margin anywhere from 1.5 mm9 to 3.0 mm below the tissues to develop emergence profiles and for esthetics. However, clinical data show that, whenever the cemented margin is placed anywhere below the soft tissues (even 1 mm), cement remnants will always remain.10 Cement remnants have been shown to result in a peri-implant disease an average of 3 years after the crown has been cemented, meaning the failure may not become apparent for some time.
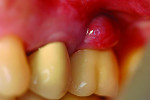
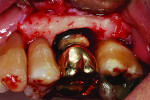
The cement itself is problematic. Manufacturers compete to produce the most adhesive materials11 bonding to substances such as zirconia, titanium, gold, and PEEK, with little regard for the negative effect of not being able to remove the excess cement residue. Cements can also directly affect the implant site, leading to failure12 (Figure 3 through Figure 6). Few, if any, are tested for their antibacterial properties, specifically for bacteria that cause peri-implant disease.13 Some cements cause allergic responses, which may result in implant rejection. Splinters of cements have been found within the soft tissues where they trigger foreign-body responses that produce inflammation. Some commonly used cements even affect the implant by corroding the titanium implant surface, which also causes rejection. None of these responses are seen with teeth because the soft tissue attachment is tight and protective, dentine is difficult to adhere to, and the cemented margins are generally placed shallower than other implant restorations.
To add to the complexity of the cemented implant restoration itself, dentists are not trained on how much cement is needed (open any textbook showing cementation and evaluate how much cement is extruded during the process). The author conducted a study that examined more than 400 dentists and how much cement they placed within a single-unit crown.14 Many dentists were placing 17 times more cement than is required for the die-relief space that technicians provide. No standards are available for where or how to place the cement so that the “flow” is controlled. However, the dental implant is a medical device that behaves differently than the body part it replaces, and dental teams must fully understand these issues. Interestingly, no protocols to date indicate which type of cement to use, how much, or where to place the cement.15,16
How a Technician Can Help
A technician should have a clear understanding that the implant surgical placement should be close to perfect. Controlling depth, site, and angulation of the implant prevents restorative compromise later. Developing a restoratively driven plan and using a surgical guide are imperative.
Restorative design must be carefully considered. The site of the margins must be controlled and the effects of subgingival placement weighed against health concerns.
Abutment type and design may promote cement extrusion directly onto the implant.17
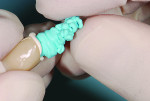
Cementation process must be controlled, and the dentist should be directed as to how much cement to use. This can be easily achieved by providing a copy polyvinyl siloxane (PVS) abutment that allows extra cement to be extruded out of the crown extra-orally before it is seated18 (Figure 7 and Figure 8).
Consider fabricating a screw-retained restoration, which would eliminate these issues completely, yet still lead to a beautiful restoration.19
These all require a team effort with the technician, surgeon, and restorative dentist each understanding their own challenges and respecting those of their colleagues.
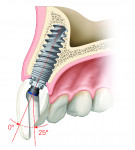
The implant industry is also developing new solutions to address these issues. One such innovative design is the angulated screw channel (ASC) abutment. This allows abutment angulation changes of up to 25 degrees, so screw-retained options can be readily used20 (Figure 9 and Figure 10).
All still have some disadvantages associated with them (usually time or economics). Remember, the dental implant is not as perfect as nature’s original tooth. If the health of the patient is considered paramount, it’s worth going that extra step.
Chandur Wadhwani, BDS, MSD, owns Northwest Prosthodontics in Bellevue, WA. He is author and editor of Cementation in Dental Implantology: An evidence based guide, published by Springer Medical Press.
See the May issue of Inside Dental Technology for part II of this article, a laboratory technician’s perspective on this case.
Reference List
1. Academy report: Peri-implant mucositis and peri-implantitis:a current understanding of their diagnosis and clinical implications. J Periodontol. 2013;84:436-43
2. Taylor TD, Agar JR. Twenty years of progress in implant prosthodontics. J. Prosthet dent 2002;88:89-95
3. Weber HP, et al. Peri-implant soft tissue health surrounding cement- and screw-retained implant restorations: A multi-center, 3 year prospective study. Clin oral Implants Res 2006;17:375-9
4. Wismeijer D et al. Consensus statements and recommended clinical procedures regarding restorative materials and techniques for implant dentistry. Int J Oral Maxillofac Implants. 2014;29 Suppl:137-40.
5. Wittneben et al. Clinical performance of screw- versus cement-retained fixed implant-supported reconstructions--a systematic review. Int J Oral Maxillofac Implants. 2014;29 Suppl:84-98
6. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. j Periodontol 2009; 80:1388-92
7. Donovan et al. Annual review of selected scientific literature: Report of the Committee on Scientific Investigation of the American Academy of Restorative Dentistry. J Prosthet Dent. 2014 Nov;112(5):1038-87.
8. Wadhwani C ed. Cementation in Dental implantology: An evidence based guide. Springer medical press 2015; 2-9
9. Rose LF, Mealey BL, Genco RJ, Cohen WD. Periodontics, medicine, surgery and implants. Philadelphia: Elsevier Mosby; 2004. p. 612–3.
10. Higginbottom F et al. Prosthetic management of implants in the esthetic zone. Int J Oral Maxillofac Implants. 2004;19(Suppl):62–72.
11. Linkevicius T et al. The influence of the cementation margin position on the amount of undetected cement. A prospective clinical study. Clin Oral Implants Res. 2013a;24:71–6.
12. Garg P, Pujari M, Prithviraj DR, Khare S. Retentiveness of various luting agents used with implant-supported prosthesis: an in vitro study. J Oral Implantol. 2014 Dec;40(6):649-54
13. Wadhwani CP. Peri-implant disease and cemented implant restorations: a multifactorial etiology. Compend Contin Educ Dent. 2013
14. Raval NC, Wadhwani CP, Jain S, Darveau RP. The Interaction of Implant Luting Cements and Oral Bacteria Linked to Peri-Implant Disease: An In Vitro Analysis of Planktonic and Biofilm Growth - A Preliminary Study. Clin Implant Dent Relat Res. 2014 Jun 6
15. Wadhwani C. et al. Cement application techniques in luting implant-supported crowns: a quantitative and qualitative survey. Int J Oral Maxillofac Implants. 2012 Jul-Aug;27(4):859-64.
16. Wadhwani C, Goodwin S, Chung KH. Cementing an Implant Crown: A Novel Measurement System Using Computational Fluid Dynamics Approach. Clin Implant Dent Relat Res. 2014 Sep 5
17. Wadhwani CP, Chung KH The role of cements in dental implant success, Part 2. Dent Today. 2013 Jun;32(6):46, 48-51
18. Wadhwani C ed. Cementation in Dental implantology: An evidence based guide. Springer medical press 2015; 10-14
19. Wadhwani C. Technique for controlling the cement for an implant crown. J prosthet Dent 2009;102:57-8
20. Wadhwani et al. An esthetic solution to the screw retained implant restoration: introduction to the implant crown adhesive plug- Clinical report. J. Esthet. Restor dent. 2001; 23:138-45
21. Wadhwani C. Technique for controlling the cement for an implant crown. J prosthet Dent 2009;102:136-7
Disclaimer: The statements and opinions contained in the preceding material are not of the editors, publisher, or the Editorial Board of Inside Dental Technology.
For more information, contact:
Nobel Biocare
800-322-5001
nobelbiocare.com