Implant-Related Gingival Recession: Pilot Case Series Presents Novel Technique and Scoring Template
Abd El Salam El Askary, BDS; Noha A. Ghallab, MD; Shuh-Chern Tan, BDS; Paul S. Rosen, DMD, MS; and Ahmad Shawkat, BDS, MS, PhD
Abstract:
This article introduces a novel protocol for the predictable treatment of Class II division 2 implant-related gingival recession and presents an innovative acrylic template for scoring the peri-implant soft-tissue gain, used before and after treatment. Ten patients with Class II division 2 single-implant–related gingival recession received combined double-papillary flap approximation and rotated subepithelial connective tissue grafting from the palate, along with any preferred optimal grafting technique that suits the type of preexisting defect. Clinical gingival recession was recorded using a scoring template at 4, 6, and 9 months postoperatively. At the end of the 9-month follow-up period, 80% of the cases showed improved soft-tissue coverage; two patients showed significant wound complications that were related to poor home-care measures. The scoring method used can be considered a diagnostic and prognostic tool for better understanding of implant-related gingival recession.
Dental implants are considered the best predictable treatment option for patients seeking replacement of missing teeth, especially in the esthetic zone, because they provide the most satisfactory functional and esthetic results compared with other treatment modalities.1 This, in turn, has increased patients’ expectations for optimal esthetics, which makes the procedure more challenging for the clinician. Marginal soft-tissue stability is considered a significant factor for achieving an esthetic outcome with implant-supported restorations,2 for which a stable architecture of the peri-implant soft tissues plays a pivotal role.
Many authors have reported variable degrees of implant-related mid-facial gingival recession ranging from 0.6 mm to 0.9 mm.3-9 This issue is affected by multiple factors, which may be either biologically or technically related.10,11 The biologic factors include labial-plate thickness, tissue phenotype, and postregenerative resorption. They are usually caused by physiologic reactions leading to a change in the level of gingival margin or bone level around dental implants. A relationship exists between the thickness of the labial plate bone and eventual gingival recession around dental implants.12 The endosteal inner surface of the labial plate of bone is mainly formed from bundle bone that begins resorption after tooth extraction, thus a marked loss of bone volume and recession of peri-implant soft tissue will soon follow.13 The thickness of the labial plate of bone is affected by both the extent of protrusion of the maxillary anterior teeth14 and the facial profile of the patient.15 Tissue phenotype has a great impact on the esthetic outcome of the dental implant and the increased prevalence of gingival recession. Thin-tissue phenotype is usually accompanied by a high-scalloped and fenestrated labial plate of bone, which might increase the risk for implant-related gingival recession (apical migration).
Technical factors can be described as the iatrogenic causes of gingival recession related to dental implants. These are usually the factors that might lead to recession of the peri-implant soft tissues that are within the clinician’s control and are largely avoidable. Technical factors described in the literature that might affect peri-implant soft tissue include implant diameter, placement, timing of final restoration delivery, biologic width violation, excess cement, and alcohol contamination. In the past, using an implant diameter close to the size of the extracted tooth was routine when replacing teeth in the anterior region. This approach was used to reduce the discrepancy in width between the implant fixture and the neck of the implant crown in an attempt to mimic natural teeth emergence. Later, this was proven to violate the labial plate of bone, causing resorption.16 Saadoun and Touati12 reported that soft-tissue recession around a wide-diameter implant (≥5 mm) averaged 1.58 mm compared with 0.57 mm around a standard-diameter implant (<5 mm). While the wider-diameter platform should provide an anatomically correct emergence profile, it may be more prudent to use standard-diameter implants in the esthetic zone.12
Another contributing factor is a strong relationship between the implant being in a labio-palatal position and the apical migration of the related soft tissue. The more labial the implant is, the further the recession of the related soft tissue occurs as a result of the approximation or pressure to the labial plate of bone. This, in turn, leads to bone resorption due to pressure from the implant.17 The time from the implant placement until final restoration delivery can also lead to implant-related gingival recession. At least 4 weeks, especially for the esthetic zone, will provide the clinician with the final gingival margin level after soft-tissue remodeling.18 Premature delivery of the prosthesis will lead to recession of the gingival tissue as the peri-implant soft tissue continues to remodel. Hence, to obtain a stable soft-tissue margin, long-term use of a provisional restoration should precede insertion of the final prosthesis. This will help the maturation of the soft tissue during provisionalization, thus minimizing the tendency for marginal exposure of the final implant-supported restoration.19
Cochran et al20 proposed a minimum of 3 mm of peri-implant mucosa, referred to as the biologic width, is required for a stable epithelial connective tissue attachment to form and serves as a protective mechanism for the underlying bone.21 The biologic width is provided by the final position of the remodeled bone; 2 mm to 3 mm apical to the implant-abutment interface in two-piece implants22 can also be a critical factor, as it is required to enable a proper epithelial and connective tissue attachment without any violation from an existing prosthesis. If the clinician does not respect this soft-tissue dimension, bone resorption will occur as a biologic response from the tissues to ensure the reestablishment of attachment with an appropriate biologic width.23,24 Violation of biologic width can occur through impression-making, excessive cement, or a faulty prosthetic margin (too deep). Although a thick gingival phenotype provides better forgiveness, it too must be carefully considered because no actual barrier is protecting the crestal bone around the dental implant,25 which will increase the risks for bone resorption and gingival recession.26
The treatment of implant-related recession has been much less studied than the causes of it.2 Some case-report studies describe the use of the connective tissue graft27,28 or the acellular dermal matrix graft29 techniques to correct soft-tissue defects on implant sites. One prospective cohort study3 evaluated the outcome of implant-related recession coverage around single-implant restorations. The authors concluded that a clinically significant improvement of implant-related recession was obtained with a combination of coronally positioned flap and connective tissue graft, but complete “recession” coverage at implant sites was not possible.
The aim of the current prospective study was to apply a novel modified surgical approach to treat implant-related gingival recession and to evaluate the outcome of soft-tissue coverage 9 months after the surgical intervention.
Materials and Methods
The current investigation was proposed to present an original protocol for the treatment of Class II division 2 implant-related gingival recession. In addition, this study introduced an innovative scoring method for assessing the level of peri-implant soft-tissue mid-buccal gain, done before and after treatment, using a custom-fabricated acrylic resin template.
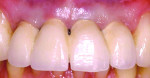
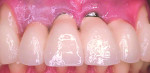
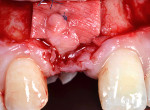
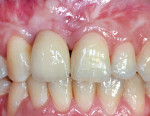
Mid-facial gingival recession may occur due to apical migration of implant-related soft tissues 1 year post-implant restoration, especially in cases with thin-tissue phenotype (Figure 1 and Figure 2). Mid-facial implant-related gingival recession is measured from the deepest point in mid-facial curve to the soft-tissue margin around the implant-supported restoration.
Study Population
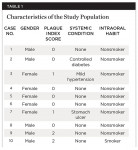
Ten participants (five women and five men) between 24 and 63 years old presented with Class II division 2 gingival recession around restored dental implants. All participants were recruited from the Institute of Implantology Education in Alexandria, Egypt. After explaining the study, researchers asked each patient to sign informed consent forms. Comprehensive medical histories were obtained, according to the detailed questionnaire of the modified Cornell Medical Index.30 Most participants were free of any untreated systemic disease, although one had controlled diabetes mellitus, one had mild hypertension, and one had received treatment for a stomach ulcer. Diabetes control was measured by a health questionnaire, three fasting blood glucose tests on weekly intervals, and glycosylated hemoglobin assessment. All patients except one were nonsmokers. This particular patient started the study as a nonsmoker and began smoking after the surgical intervention had been performed (Table 1).
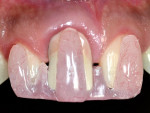
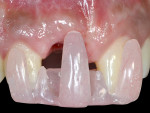
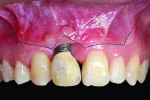
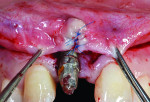
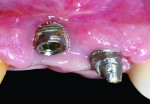
Exclusion criteria included smoking and alcoholism. All 10 patients received preoperative clinical and radiographic examinations (cone-beam computed tomography [CBCT]), and baseline plaque index scores31 were documented. Baseline markings of gingival recession were recorded using a specially designed acrylic template (Figure 3 and Figure 4). Measurements were again recorded at 4, 6, and 9 months postoperatively.
Novel Scoring Template
The present study demonstrates a simple novel device that focuses on monitoring the improvement of implant-related gingival recession, used before and after treatment (Figure 3 and Figure 4). The device consists of a custom-designed template that has an acrylic extension resting on the incisal edges of the maxillary teeth adjacent to the dental implant and protruding to the mid-buccal gingival contour of the affected implant-supported crown and its adjacent teeth. The template is used to record implant-related gingival recession, as it reflects the amount of existing recession prior to treatment. It also indicates the amount of soft-tissue correction needed, which is represented by a line connecting the mid-buccal point of the two adjacent teeth. This method is subjective because it involves an observer who grades the amount of the recession on a 0-to-2 scoring system.
After treatment of the gingival recession, the acrylic template is used again to compare the difference in the levels of the gingival margin. This is assessed by measuring the deepest point in the mid-buccal curve around the old implant-supported restoration and comparing it with the soft-tissue margin around the new implant-supported restoration after the completion of treatment.
Surgical Procedure
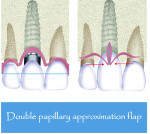
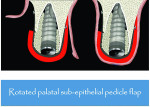
This study introduces a combined approach for the treatment of Class II division 2 implant-related gingival recession that includes double-papillary flap approximation and a rotated palatal subepithelial connective tissue pedicle graft (Figure 5 and Figure 6) used together covering any particulated or autogenous bone graft material. All surgeries were performed under local anesthesia using 2% Xylocaine (DENTSPLY, www.dentsply.com) with adrenaline after removing the existing prosthesis.
In the authors’ opinion, the currently proposed procedure should be performed in three stages: soft-tissue preparation, implant removal and replacement with a new implant, and, finally, bone grafting.
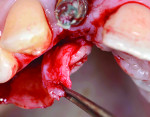
The soft-tissue preparation includes double-papillary flap approximation and dissection of the palatal pedicle connective tissue graft preparation. Double papillary flap was performed buccally,32 and a No. 15 blade was used to make a V-shaped incision labially following the outline of the gingival recession (Figure 7). This incision provided a fresh wound surface for tissue approximation. Two horizontal incisions were made on the adjacent mesial and distal interdental papilla coronally parallel to the cementoenamel junction with a No. 15 blade to allow better approximation of the flap. Two releasing incisions were made obliquely at the line angles of the adjacent teeth; these incisions were extended beyond the mucogingival junction. Periosteal fenestrations were performed for tension-free flap closure.
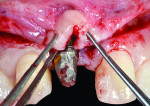
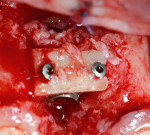
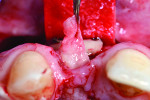
The V-section was then raised together with the buccal flap. A suture needle was passed through the outer surface of the first papilla and continued through the undersurface of the second papilla to approximate the double papillae flap using 5-0 silk interrupted sutures (Hu-Friedy, www.hu-friedy.com) (Figure 8 and Figure 9). At this juncture, a palatal flap was raised to harvest a subepithelial connective tissue graft directly opposite the recession area, which would later be rotated to the buccal recession area. As described by Khoury and Happe,33 a palatal paramarginal incision was made from the anterior region to the defect to be covered. Dissection of the mucoperiosteal flap and the underlying preparation of a subepithelial connective tissue flap to a depth of 5 mm to 8 mm were then performed. In the same manner, a sharp incision of the subepithelial tissue was then made parallel to the first incision to harvest a subepithelial connective tissue graft, but it was left attached in the anterior region as a pedicle (Figure 10 through Figure 13).
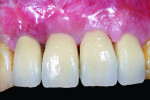
In the currently presented protocol, the existing restorations were removed along with the connected final abutment. This was followed by inserting a new implant (Laser-Lok®, Biohorizons, www.biohorizons.com) at the same time, and the resultant osseous defect around the new implant was grafted with particulated autogenous bone graft mix. The bone graft mix was formed of autogenous bone chips harvested from the operating site using bone scrapers (Hu-Friedy) and deproteinized bovine bone xenografts (Bio-Oss®, Geistlich Pharma, www.geistlich-pharma.com). The particulated bone graft was then stabilized and covered with a poly-D-L-lactic acid (PDLLA) membrane (SonicWeld technology, KLS Martin, https://klsmartin.com) and four thermal screws. The palatal subepithelial connective tissue pedicle was then elevated and rotated incisally and labially to cover the bone graft and sutured to the shield membrane (SonicWeld technology, KLS Martin) using 6-0 Coated VICRYL™ (polyglactin 910) Suture (Ethicon,www.ethicon.com) (Figure 14 and Figure 15). Because only a subepithelial connective tissue pedicle was removed, the palatal wound at the donor site could be totally closed and final closure of the labial and palatal flaps performed by 4-0 silk sling sutures (Hu-Friedy). Postoperative radiographs were taken using CBCT. The new implant was left submerged during the healing period, and a Maryland bridge (resin-bonded bridge) was used for provisional purposes in most of the cases. Figure 16 depicts the placement of a final restoration and complete coverage of the gingival recession 6 months after implant placement.
Postoperative instructions given to the patients included gentle brushing in the areas near the surgical site. Patients were advised to refrain from brushing at the grafted site for 3 weeks and to rinse with 0.12% chlorhexidine mouthrinse (Antiseptol, Kahira Pharmaceuticals, kahira-pharma.com) twice a day for 1 week. Amoxicillin (500 mg) (E-mox 500 mg Cap., Egyptian Int. Pharmaceutical Industrial Co., A.R.E) three times a day was prescribed for 5 days to prevent postoperative infection.
Statistical Analysis
Data analyses of the clinical measurements and patient characteristics were performed by using Fisher’s exact test, Monte Carlo methods, and repeated ANOVA measures. All data were processed with a computerized statistical package, IBM® SPSS® Statistics Version 20 for Windows.
Results
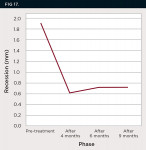
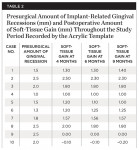
All patients, each with labial implant-related gingival recession at a single-standing implant, were followed for 4, 6, and 9 months after surgical recession coverage. Table 2 presents the presurgical amount of implant-related gingival recessions (mm) and postoperative amount of soft-tissue gain (mm) throughout the study period. Moreover, the mean ± SD amount of implant-related gingival recession (mm) at different follow-up periods is represented in Table 3 and Figure 17. On average, the mean ± SD baseline gingival recession was 1.9 mm ± 0.5 mm, which decreased significantly (P < .05) during the experimental study to reach 0.6 mm ± 0.9 mm after 4 months. Furthermore, at 6 and 9 months postoperative, the mean ± SD gingival recession observed was 0.7 mm ± 0.9 mm, which was also statistically significant compared to baseline values (P < .05). On comparing gingival recession values at 4, 6, and 9 months postoperative, no statistically significant difference was evident. These results showed that the amount of soft tissue gained after the surgical procedure did not deteriorate and showed good stability during the follow-up period.
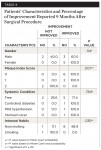
At the end of the 9-month follow-up period, all cases except two showed improved soft-tissue coverage of the recession area (Table 4). Of the two patients who failed to show soft-tissue gain, one was a smoker and the other had a higher plaque index score of 2. At the end of the study phase, 80% of the patients had improved soft-tissue coverage in response to the surgical intervention.
Discussion
Long-term clinical studies have shown that functional osseointegration is a predictable outcome when endosseous implants are placed to treat missing teeth.34 However, patients expect not only the ability to function long term with their implants, but also to enjoy the esthetics of the treatment outcome. This may explain the growing interest by scientists for soft-tissue dynamics, objective esthetic ratings, and patient-centered outcomes.35
Although implant success measured through fixture osseointegration and restoration of function is high, attaining predictable esthetic results is not simple.36,37 Though the esthetic zone has been extensively studied in the past two decades, anterior restorations continue to pose a substantial challenge to the dental team. The objective is to influence each element of treatment, starting with management of hard and soft tissues that surround the implants, and the selected implant components to achieve ultimate esthetics and function.38
Soft-tissue grafts have been successfully used in periodontal treatment for many years to cover areas of root recession and alveolar ridge reconstruction.39,40 Although the double papilla approximation is technique sensitive, it has shown good results for treating isolated recessions.41 The double papilla approximation evolved from treating defects in which tissues adjacent or apical to the defect alone may be inadequate for grafting purposes.32 The partial-thickness double-papilla pedicle-graft technique was first proposed by Cohen and Ross42 who reported a more than 85% success rate in covering denuded roots. Nelson43 proposed a technique that combines a free-connective tissue graft with a full-thickness double papilla graft. The double papilla pedicle graft is most appropriate in those cases in which esthetics demand a close tissue-color match and the papillae are large and have shallow gingival grooves.44
It has been suggested that the use of pedicle grafts present a much more favorable prognosis than free grafts45 because an important part of the blood supply to the flap is maintained during and after the procedure, which is consistent with the present study. Burkhardt et al3 concluded that a subepithelial connective tissue graft in conjunction with a coronally advanced flap could improve the condition of the soft-tissue recession around dental implants; however, complete coverage was not achieved. Immediately after surgery, all sites presented recession coverage of >100%. These positive outcomes were not maintained after 6 months. One month postoperative, a significant decrease of coverage to 75% was observed. Further decreases were reported after 3 and 6 months with 70% and 66%, respectively, of the initial recession covered.
In contrast, Zucchelli et al2 reported a mean coverage of 96.3% and 100% coverage in 75% of the treated sites 1 year after final crown delivery. This was probably due to the fact that 1 month prior to surgery, they removed the implant crowns and reshaped and polished the underlying abutments. Moreover, the newly fabricated provisional crowns were removed at the time of surgery. As a result of these prosthetic procedures, more room was created for the soft-tissue graft to be placed over the implant-abutment interface, and a better adaptation between the graft and the smoothed abutment surface was obtained. This may have contributed to the better clinical outcomes reported. In a 1-year prospective study, Cosyn et al46 presented the outcomes of 22 immediately placed implants. Two cases showed severe mid-facial recession of 1.5 mm and 2.0 mm after 3 months and were corrected by a connective tissue graft. After 1 year, the recession measured was 0.5 mm in both cases.
Although conventional subepithelial connective tissue grafts can improve the buccal aspect of compromised sites, coronal gain remains unpredictable. The pediculated connective tissue graft is an excellent technique that can be used for vertical and labial augmentation of soft tissue and can be employed to improve unesthetic soft-tissue structures around implants. Khoury and Happe35 demonstrated that, in cases of immediate implants, the palatal subepithelial connective tissue pedicle is advantageous in combination with different bone augmentation methods for the maxilla. The pedicle creates an additional soft-tissue layer covering the grafted bone and/or membranes and also provides an underlying tissue blanket for the double papilla approximated flaps. Moreover, the authors underscored the factors considered for the long-term success of subepithelial connective tissue grafts, including primary fixation of the graft to the underlying bed and the possibility of revascularization and revitalization from the receptor site. Collectively, the present study supports the data previously mentioned above.
Although various techniques contribute to the improvement of peri-implant soft tissue, it is a prerequisite that sufficient bone be available to support the augmented tissue.47 In the past two decades, research investigators have suggested a combination of guided-bone regeneration principles with allogeneic and xenogeneic bone substitutes for the treatment of such defects.44 Biodegradable membranes composed of dura mater, poly-L-lactic acid, polyglycolic acid, polyurethane, and collagen have been introduced to avoid a need for a second surgery for membrane removal.48,49 In a recent systematic review, Khojasteh et al50 critically appraised the available evidence regarding the effectiveness of barrier membranes for bone augmentation. According to the meta-analysis, barrier membranes may have a benefit in the treatment of vertical bone defects around implants. With respect to precision of the techniques used, it has been suggested that membrane stability should be maintained during bone regeneration. Adeyemo et al51 demonstrated this when they observed significant bone resorption following membrane instability.
In their review regarding regenerative barriers in immediate implant placement, El Helow and El Askary52 said the decision to use grafts under barrier membranes in immediate implantation should be left to clinical judgment. Most bony voids are 3-dimensional, whereas all membranes yet used are 2-dimensional. While shaping the membrane to a 3-dimensional configuration is time consuming and requires a sort of mechanical memory, the use of grafts under barriers prevents membranes from springing into the defect.53,54 In addition, the superiority of combining grafts with barriers compared to the single use of either of them has been previously advocated.55 Based on the above-mentioned studies, PDLLA bioresorbable membrane was the barrier of choice in the current study because of its biocompatibility, ease of handling, and the elimination of the need for a second reentry surgery.
Several types of bone grafts have been used under barriers in immediate implantation in the literature, including demineralized and mineralized freeze-dried bone allograft, deproteinized porcine bone particles, resorbable hydroxyapatite, autogenous bone chips, bioactive glass, and porous bone mineral matrix xenograft.56-59 Meanwhile, the use of autogenous bone grafts has shown superiority in providing better quantity and quality of bone.60 In their study, El Askary and Pipco38 reported that they prefer to use autogenous bone grafts in situations of extensive bone loss. Subsequently, the present study used a mixture of particulated autogenous bone graft and porous bone mineral matrix xenograft (Bio-Oss) to fill the bony defects around the newly placed implant.
Another peculiarity of the novel surgical technique presented in this study was the coronal placement of the double papilla pedicle flap that allowed for a greater coronal advancement of the covering flap. This might have contributed to improved soft-tissue coverage outcome61 and permitted maintenance of the subepithelial connective tissue pedicle with the underlying bone graft and membrane covered by the primary flap. Furthermore, the lack of pedicle exposure might contribute to the favorable esthetic outcome reported in this investigation.
Although patient selection in this study was not highly restrictive, with treatments performed on patients with controlled diabetes, mild hypertension, and a stomach ulcer, the observed success rate demonstrated that these conditions did not affect the treatment outcomes for implant-related gingival deficiencies. Patients who are not likely to respond well are smokers and those with high-plaque-score indices.
It is necessary to determine the amount of tissue gain after implant-related gingival recession surgery, measuring it from its baseline, or to record the amount of gingival recession following implant placement. Cabello et al62 observed the soft-tissue level changes in relation to immediate implant placement. In their study, they used a rigid acrylic stent overlapping the incisal edges of the adjacent teeth with three reference dimples placed in the stent corresponding to the mesial and distal interdental papilla and the level of the gingival zenith. An electronic caliber was used to measure the distance approximated to the tenth of a millimeter. A simpler method is to use the acrylic template as described in the present article. After gingival recession treatment, the acrylic template can be placed again and the difference in the gingival margin levels compared. This technique focuses mainly on monitoring implant-related gingival recession.
Conclusions
Identifying the tissue phenotype and the reason for the implant-related gingival recession is a vital part of treatment. To maximize success of treating gingival recession, the authors recommend using routine subepithelial connective tissue grafts with implant procedures in thin-tissue phenotype cases, as their usage has shown a remarkable increase in the overall buccal tissue volume63 and routine doubling of thickness in cases involving thin labial plate of bone. Additionally, this approach enables long-term provisional restoration for 3 months and the use of platform-switching implants,64 and ensures at least 3 mm of keratinized tissue width. Soft-tissue solutions alone usually offer less consistent success rates for the treatment of Class I implant-related gingival recession. Also, using coronal repositioning flaps to treat implant-related gingival recession does not offer any clinical value and would complicate the overall prognoses of the treatment. Furthermore, whenever soft-tissue surgery is performed, soft-tissue scarring and stiffness should be anticipated. This explains the authors’ preference for the use of the nonstaged grafting approach whereby soft and hard tissue were augmented and repaired at one time, and the inflammatory tissue in the site was completely removed. This solution also minimizes the number of surgeries and helps provide better patient acceptance.
Further prospective studies with larger samples and longer follow-ups are warranted to confirm the results of the present findings. This research opens the door to explore this novel surgical procedure as a promising strategy for treatment of implant-related gingival recession.
About the Authors
Abd El Salam El Askary, BDS
Visiting Lecturer
New York University
New York, New York
Private Practice
Alexandria, Egypt
Noha A. Ghallab, MD
Associate Professor
Periodontology and Oral Medicine
Faculty of Dental Medicine, Cairo University
Cairo, Egypt
Shuh-Chern Tan, BDS
Private Practice
Singapore
Paul S. Rosen, DMD, MS
Clinical Associate Professor of Periodontics
University of Maryland Dental School
Baltimore, Maryland
Private Practice
Yardley, Pennsylvania
Ahmad Shawkat, BDS, MS, PhD
Lecturer
Department of Oral Medicine
Oral Diagnosis, and Periodontology
Faculty of Damanhur University
Damanhur, Egypt
References
1. Belser UC, Buser D, Hess D, et al. Aesthetic implant restorations in partially edentulous patients—a critical appraisal. Periodontol 2000. 1998;17:132-150.
2. Zucchelli G, Mazzotti C, Mounssif I, et al. A novel surgical-prosthetic approach for soft tissue dehiscence coverage around single implant. Clin Oral Implants Res. 2013;24(9):957-962.
3. Burkhardt R, Joss A, Lang NP. Soft tissue dehiscence coverage around endosseous implants: a prospective cohort study. Clin Oral Implants Res. 2008;19(5):451-457.
4. Cardaropoli G, Lekholm U, Wennstrom JL. Tissue alterations at implant-supported single-tooth replacements: a 1-year prospective clinical study. Clin Oral Implants Res. 2006;17(2):165-171.
5. Evans CD, Chen ST. Esthetic outcomes of immediate implant placements. Clin Oral Implants Res. 2008;19(1):73-80.
6. Small PN, Tarnow DP. Gingival recession around implants: a 1-year longitudinal prospective study. Int J Oral Maxillofac Implants. 2000;15(4):527-532.
7. Juodzbalys G, Wang HL. Soft and hard tissue assessment of immediate implant placement: a case series. Clin Oral Implants Res. 2007;18(2):237-243.
8. Kan JY, Rungcharassaeng K, Lozada J. Immediate placement and provisionalization of maxillary anterior single implants: 1-year prospective study. Int J Oral Maxillofac Implants. 2003;18(1):31-39.
9. De Rouck T, Collys K, Wyn I, Cosyn J. Instant provisionalization of immediate single-tooth implants is essential to optimize esthetic treatment outcome. Clin Oral Implants Res. 2009;20(6):566-570.
10. Sorni-Bröker M, Peñarrocha-Diago M. Factors that influence the position of the peri-implant soft tissues: a review. Med Oral Patol Oral Cir Bucal. 2009;14(9):e475-e479.
11. Nisapakultorn K, Suphanantachat S, Silkosessak O, Rattanamongkolgul S. Factors affecting soft tissue level around anterior maxillary single-tooth implants. Clin Oral Implants Res. 2010;21(6):662-670.
12. Saadoun AP, Touati B. Soft tissue recession around implants: is it still unavoidable?—Part I. Pract Proced Aesthet Dent. 2007;19(1):55-62.
13. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212-218.
14. Yu Q, Pan XG, Ji GP, Shen G. The association between lower incisal inclination and morphology of the supporting alveolar bone—a cone-beam CT study. Int J Oral Sci. 2009;1(4):217-223.
15. Gracco A, Lombardo L, Mancuso G, et al. Upper incisor position and bony support in untreated patients as seen on CBCT. Angle Orthod. 2009;79(4):692-702.
16. Buser D, Martin W, Belser UC. Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants. 2004;19 suppl:43-61.
17. Al-Sabbagh M. Implants in the esthetic zone. Dent Clin North Am. 2006;50(3):391-407, vi.
18. Kinsel RP, Capoferri D. A simplified method to develop optimal gingival contours for the single implant-supported, metal-ceramic crown in the aesthetic zone. Pract Proced Aesthet Dent. 2008;20(4):231-236.
19. Lazzara RJ. Effect of implant position on implant restoration design. J Esthet Dent. 1993;5(6):265-269.
20. Cochran DL, Hermann JS, Schenk RK, et al. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 1997;68(2):186-198.
21. Lindhe J, Berglundh T, Ericsson I, et al. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3(1):9-16.
22. Hermann JS, Buser D, Schenk RK, et al. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000;11(1):1-11.
23. Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996;23(10):971-973.
24. Scarano A, Assenza B, Piattelli M, et al. Interimplant distance and crestal bone resorption: a histologic study in the canine mandible. Clin Implant Dent Relat Res. 2004;6(3):150-156.
25. Hermann JS, Buser D, Schenk RK, Cochran DL. Crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged and submerged implants in the canine mandible. J Periodontol. 2000;71(9):1412-1424.
26. Kinsel RP, Lamb RE. Tissue-directed placement of dental implants in the esthetic zone for long-term biologic synergy: a clinical report. Int J Oral Maxillofac Implants. 2005;20(6):913-922.
27. Shibli JA, d’avila S, Marcantonio E Jr. Connective tissue graft to correct peri-implant soft tissue margin: a clinical report. J Prosthet Dent. 2004;91(2):119-122.
28. Lai YL, Chen HL, Chang LY, Lee SY. Resubmergence technique for the management of soft tissue recession around an implant: case report. Int J Oral Maxillofac Implants. 2010;25(1):201-204.
29. Mareque-Bueno S. A novel surgical procedure for coronally repositioning of the buccal implant mucosa using acellular dermal matrix: a case report. J Periodontol. 2011;82(1):151-156.
30. Abramson JH. The cornell medical index as an epidemiological tool. Am J Public Health Nations Health. 1966;56(2):287-298.
31. Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121-135.
32. Yalamanchili PS, Pavithra D, Potluri S, Arunima PR. Root coverage using double papilla preservation flap: a case report. J Int Oral Health. 2014;6(6):82-84.
33. Khoury F, Happe A. The palatal subepithelial connective tissue flap method for soft tissue management to cover maxillary defects: a clinical report. Int J Oral Maxillofac Implants. 2000;15(3):415-418.
34. Esposito M, Grusovin MG, Chew YS, et al. Interventions for replacing missing teeth: 1- versus 2-stage implant placement. Cochrane Database Syst Rev. 2009;(3):CD006698.
35. Cosyn J, Hooghe N, De Bruyn H. A systematic review on the frequency of advanced recession following single immediate implant treatment. J Clin Periodontol. 2012;39(6):582-589.
36. Levine RA, Huynh-Ba G, Cochran DL. Soft tissue augmentation procedures for mucogingival defects in esthetic sites. Int J Oral Maxillofac Implants. 2014;29 suppl:155-185.
37. Kazor CE, Al-Shammari K, Sarment DP, et al. Implant plastic surgery: a review and rationale. J Oral Implantol. 2004;30(4):240-254.
38. El-Askary AS, Pipco DJ. Autogenous and allogenous bone grafting techniques to maximize esthetics: a clinical report. J Prosthet Dent. 2000;83(2):153-157.
39. Langer B, Calagna L. The subepithelial connective tissue graft. J Prosthet Dent. 1980;44(4):363-367.
40. Novaes AB Jr, Novaes AB. Soft tissue management for primary closure in guided bone regeneration: surgical technique and case report. Int J Oral Maxillofac Implants. 1997;12(1):84-87.
41. Shetty NJ. Double papilla repositioned flap for the treatment of isolated recession - A case report. Singapore Dent J. 2013;34(1):25-27.
42. Cohen DW, Ross SE. The double papillae repositioned flap in periodontal therapy. J Periodontol. 1968;39(2):65-70.
43. Nelson SW. The subpedicle connective tissue graft. A bilaminar reconstructive procedure for the coverage of denuded root surfaces. J Periodontol. 1987;58(2):95-102.
44. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81(5):672-676.
45. Rosenquist B. A comparison of various methods of soft tissue management following the immediate placement of implants into extraction sockets. Int J Oral Maxillofac Implants. 1997;12(1):43-51.
46. Cosyn J, De Bruyn H, Cleymaet R. Soft tissue preservation and pink aesthetics around single immediate implant restorations: a 1-year prospective study. Clin Implant Dent Relat Res. 2013;15(6):847-857.
47. Khoury F, Happe A. Soft tissue management in oral implantology: a review of surgical techniques for shaping an esthetic and functional peri-implant soft tissue structure. Quintessence Int. 2000;31(7):483-499.
48. Jovanovic SA, Spiekermann H, Richter EJ. Bone regeneration around titanium dental implants in dehisced defect sites: a clinical study. Int J Oral Maxillofac Implants. 1992;7(2):233-245.
49. Lindhe J, Nyman S, Lang N. Treatment planning. In: Lindhe J, Karring T, Lang N, eds. Clinical Periodontology and Implant Dentistry. 4th ed. Oxford: Blackwell Munksgaard; 2003:414-431.
50. Khojasteh A, Soheilifar S, Mohajerani H, Nowzari H. The effectiveness of barrier membranes on bone regeneration in localized bony defects: a systematic review. Int J Oral Maxillofac Implants. 2013;28(4):1076-1089.
51. Adeyemo WL, Reuther T, Bloch W, et al. Healing of onlay mandibular bone grafts covered with collagen membrane or bovine bone substitutes: a microscopical and immunohistochemical study in the sheep. Int J Oral Maxillofac Surg. 2008;37(7):651-659.
52. El Helow K, El Askary Ael S. Regenerative barriers in immediate implant placement: a literature review. Implant Dent. 2008;17(3):360-371.
53. Kohal RJ, Mellas P, Hurzeler MB, et al. The effects of guided bone regeneration and grafting on implants placed into immediate extraction sockets. An experimental study in dogs. J Periodontol. 1998;69(8):927-937.
54. Becker W, Schenk R, Higuchi K, et al. Variations in bone regeneration adjacent to implants augmented with barrier membranes alone or with demineralized freeze-dried bone or autologous grafts: a study in dogs. Int J Oral Maxillofac Implants. 1995;10(2):143-154.
55. Tehemar S, Hanes P, Sharawy M. Enhancement of osseointegration of implants placed into extraction sockets of healthy and periodontally diseased teeth by using graft material, an ePTFE membrane, or a combination. Clin Implant Dent Relat Res. 2003;5(3):193-211.
56. Stentz WC, Mealey BL, Gunsolley JC, Waldrop TC. Effects of guided bone regeneration around commercially pure titanium and hydroxyapatite-coated dental implants. II. Histologic analysis. J Periodontol. 1997;68(10):933-949.
57. Becker W, Urist M, Becker BE, et al. Clinical and histologic observations of sites implanted with intraoral autologous bone grafts or allografts. 15 human case reports. J Periodontol. 1996;67(10):1025-1033.
58. Becker W, Clokie C, Sennerby L, et al. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol. 1998;69(4):414-421.
59. Caplanis N, Sigurdsson TJ, Rohrer MD, Wikesjo UM. Effect of allogeneic, freeze-dried, demineralized bone matrix on guided bone regeneration in supra-alveolar peri-implant defects in dogs. Int J Oral Maxillofac Implants. 1997;12(5):634-642.
60. Pikos MA. Block autografts for localized ridge augmentation: Part I. The posterior maxilla. Implant Dent. 1999;8(3):279-285.
61. Pini Prato GP, Baldi C, Nieri M, et al. Coronally advanced flap: the post-surgical position of the gingival margin is an important factor for achieving complete root coverage. J Periodontol. 2005;76(5):713-722.
62. Cabello G, Rioboo M, Fábrega JG. Immediate placement and restoration of implants in the aesthetic zone with a trimodal approach: soft tissue alterations and its relation to gingival biotype. Clin Oral Implants Res. 2013;24(10):1094-1100.
63. Grunder U. Crestal ridge width changes when placing implants at the time of tooth extraction with and without soft tissue augmentation after a healing period of 6 months: report of 24 consecutive cases. Int J Periodontics Restorative Dent. 2011;31(1):9-17.
64. Moergel M, Rocha S, Messias A, et al. Radiographic evaluation of conical tapered platform-switched implants in the posterior mandible: 1-year results of a two-center prospective study. Clin Oral Implants Res. 2016;27(6):686-693.