Case Involving CAD/CAM-Generated, Screw-Retained Bridge Demonstrates Dentistry’s Scientific Progression
Abstract
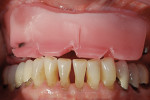
The patient in the case presented had successfully used a maxillary overdenture for more than three decades. However, the time had come for the prosthetic appliance to be replaced. This case, which involved the use of a titanium understructure that was designed and fabricated using CAD/CAM technology, illustrates how far dentistry has come since the patient first presented to the clinician some 35 years ago when dental science had not yet included such innovations as bone grafting and CT scans.
This case involved a mature female who had been a patient of the clinician for 35 years. Her case demonstrates the scientific progression of dentistry over the past three decades and the advances the industry has made with regard to treatment options.
The patient originally presented approximately 33 years ago with multiple decayed and missing teeth. She had six remaining maxillary teeth. The clinician’s goal at that time was to preserve as much of the patient’s bone as possible for future treatment, which was essential to avoid rapidity in bone loss resulting in atrophy of the ridges. The reader must keep in mind that back then science was not nearly as sophisticated as it is today and did not include bone grafting or computed tomography (CT) scans.
The six remaining teeth were endodontically treated. The post core and copings were placed in keeping with protocol and a popular method at the time, which involved the use of magnets to retain the overdenture. Copings were cast and a magnetic button was soldered to the top of the copings to maintain as low a profile as possible.
In the overdenture, five or six magnets were placed in the inner portion of the prosthesis, where the six teeth remained, and a conventional (magnetic) denture was fabricated and attached. The patient was pleased with the functionality and retention concept of preserving bone as well as the comfort level that was established. Thus, her prosthetic appliance was successful for 33 years.
With the many medical and technological advances since then involving implants and bone physiology, implant placement has become much more prolific and the procedures more advanced. Some of these innovations include autogenous bone grafing,1 bone morphogenetic proteins (BMP),2-5 platelet-enriched plasma,6-9 and the Osstell unit used to measure the stability of implants. These are just a few of the advances that have been made when it comes to understanding osseointegration. The sophisticated techniques dentistry now employs have made many procedures possible that could not have been considered 33 years earlier.
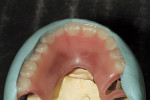
When the patient returned for treatment recently, it was determined that it was necessary to remove the remaining teeth due to excessive decay. With CT planning—a digital geometry process that generates a 3-dimensional (3-D) image of the inside of an object—and stereolithography, also known as computer-aided design/computer-aided manufacturing (CAD/CAM) or a computer-controlled laser, surgical implant placement was carried out, and the implants successfully integrated. The prosthetic protocol is outlined below.
Case Presentation
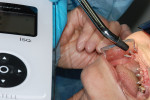
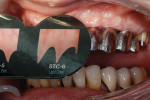
An Osstell ISQ™ implant stability unit (Osstell, www.osstell.com) was used to measure the electromagnetic field of the implants (Figure 1). The device, which determines both initial or primary stability and the stability prior to the restorative procedures (ie, secondary stability),9-14 uses the energy from the implant pegs and is attracted to a special probe. The readings received, which are called “implant stability quotients,” are used to determine the degree of bone density that has been established, bringing a new dimension of strength and stability to the design of the final restoration (Figure 2). The clinician obtains greater objectivity in determining integration of the process, because through the scientific quotient measurement, placement and assimilation are verified at the same time. The authors were thus able to determine that a fixed appliance could be used due to the uniformity of the bone.
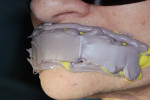
Using light- and heavy-body impression material (Aquasil Ultra LV [Light Body] and Heavy Body, DENTSPLY Caulk, www.caulk.com), the patient’s lips were measured. Because edentulous patients do not have proper lip support, the measurement must be created by the technician (Figure 3). The facial moulage results were translated (Figure 4), and the patient’s appearance was measured (Figure 5). The facial moulage is a 3-D replication of the patient’s existing features in the edentulous state. It is not a definite measure of vertical dimension but provides a 3-D concept of bone atrophy, lack of lip support, and need for added material to create a good facial profile.
In this case, the authors needed the facial moulage of the patient’s lower as well as upper facial features. Using a model of lips from the patient’s actual impression was essential in order to exactly recreate her facial shape for an accurate fit of the denture. A bite block, a laboratory procedure requirement to determine correct vertical dimension and to check centric relation of the restoration, was then employed (Figure 6). The temporary denture was measured with putty matrix for correct sizing of teeth (Figure 7). This served as a guide for the frame design.
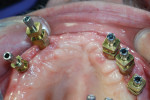
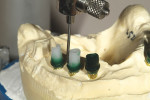
The milled UCLA abutments (a gold-plastic abutment used for waxing a custom abutment or screw-retained crown) were measured with a survey abutment to ensure proper undercut (Figure 8). After casting, the abutments were placed on the original model for a fit check with the matrix (Figure 9). After the metal framework was finished, it was tried on the original cast for a final check before cementation (Figure 10). The clinician applied a GC Pattern Resin™ (GC America Inc., www.gcamerica.com) to set the verification jig in the patient’s mouth. This very important stage checks the position of the custom abutment from the model to the mouth (Figure 11) and ensures that the bridge will fit properly; otherwise, the abutment could be turned in the wrong direction.
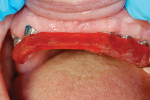
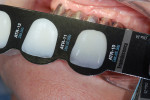
Because the technician needs to know the correct amount of translucency or transparency enamel to make lifelike, natural-looking teeth, he checked the patient’s shade and translucency using the Chairside Shade Guide™ (LSK121 Oral Prosthetics, www.lsk121.com) (Figure 12).15 Knowing that a conventional shade tab cannot adequately depict the required transparency, the clinician had previously discussed this issue with the patient, and after comparing her teeth to the Chairside Shade Guide they decided upon medium translucency, which the technician was able to apply. The titanium understructure was designed and fabricated using CAD/CAM technology (Figure 13) and was tried in the patient’s mouth for a position check. The tissue color was determined to be somewhere between light pink and light coral (Figure 14). The technician needed to mix the gingiva colors and blend translucency to achieve the correct effect. In the authors’ experience, there is no one perfect solid color that absolutely matches gingiva color. Instead, the key is to overlay it with pink porcelain to achieve the desired effect.
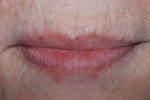
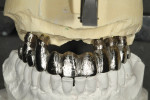
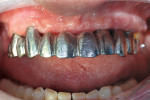
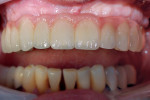
GC Initial™ Porcelain (GC America) was used to produce a good color match. During the second build-up, porcelain and a pink color were blended to create the optimal shade results (Figure 15). The 11-unit screw-retained bridge restoration and implant denture is shown in Figure 16. Finally, the case was inserted in the mouth for the patient to approve (Figure 17). A final, relaxed smile view showing proper lip support is seen in Figure 18.
Conclusion
This was a complicated case that utilized multiple special applications combined with the clinician’s wealth of knowledge, which was achieved over a period of 30 years of practice. In fact, the approach for this case included the combined wisdom and knowledge of the prosthodontist, oral surgeon, and dental technician. The availability of the CT scan and stereolithography technology aided the team in their decision-making process. The case duration was 2-½ years total—which the patient considered to be well worth the time and effort spent. The clinician views the case as an excellent example of current “state-of-the-art” dentistry, with outstanding results for all concerned, including the patient, who was pleased and proud of her new smile—the ultimate goal in difficult cases.
With good communication between the clinician and laboratory technician along with accurate information, the correct steps can be followed to attain the best results possible along with proper occlusal contact.
References
1. Borgonovo AE, Tommasi F, Panigalli A, et al. Use of fresh frozen bone graft in rehabilitation of maxillar atrophy. Minerva Stomatol. 2012;61(4):141-154.
2. Medtronic Receives Approval to Market INFUSE Bone Graft for Certain Oral Maxillofacial and Dental Regenerative Applications. Business Wire. March 13, 2007. https://findarticles.com/p/articles/mi_m0EIN/is_2007_March_13/ai_n27185616/. Accessed July 9, 2012.
3. Reddi AH. Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev. 1997;8(1):11-20.
4. Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50(6):1392-1406.
5. Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528-1534.
6. Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet-rich plasma: a literature review. Implant Dent. 2009;18(4):303-310.
7. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
8. Gonshor A. Technique for producing platelet-rich plasma and platelet concentrate: background and process. Int J Periodontics Restorative Dent. 2002;22(6):547-557.
9. Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-2272.
10. Meredith N, Alleyne D, Cawley P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7(3):261-267.
11. Meredith N, Book K, Friberg B, et al. Resonance frequency measurements of implant stability in vivo. A cross-sectional and longitudinal study of resonance frequency measurements on implants in the edentulous and partially dentate maxilla. Clin Oral Implants Res. 1997;8(3):226-233.
12. Friberg B, Sennerby L, Meredith N, Lekholm U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. Int J Oral Maxillofac Surg. 1999;28(4):297-303.
13. Bischof M, Nedir R, Szmukler-Moncler S, et al. Implant stability measurement of delayed and immediately loaded implants during healing. Clin Oral Implants Res. 2004;15(5):529-539.
14. Nedir R, Bischof M, Szmukler-Moncler S, et al. Predicting osseointegration by means of implant primary stability. Clin Oral Implants Res. 2004;15(5):520-528.
15. Kahng L. Smile Selection Plus CS³ Clinical Cases. Toronto, Ontario: Palmeri Publishing; 2010.
About the Authors
Joseph L. Caruso, DDS, MS
Private Practice
Chicago, Illinois
Luke S. Kahng, CDT
Owner
LSK121 Oral Prosthetics
Naperville, Illinois