Dentin Hypersensitivity: Its Inter-Relationship to Gingival Recession and Acid Erosion
Abstract
Often referred to as the “common cold of dentistry,” dentin hypersensitivity is a common oral condition that affects many patients,1 yet few of them bring this issue to the attention of either their dentist or dental hygienist. They may be unaware that they may have a diagnosable dental condition and, therefore, are unaware that there is something that can be done to stop, reduce, or prevent the pain. As the onset is often insidious, sufferers do not realize how they have developed coping strategies to minimize the discomfort, such as less or no ice in cold drinks, use of a straw to direct cold fluids away from “problem teeth,” tooth brushing with warm water, etc. Collectively these behaviors and the pain itself lead to deterioration in quality-of-life. Sufferers may feel that the problem is not serious or bothersome enough to warrant concern or attention and/or, in some cases, they fear that this dental pain could be a sign of something more serious that might require more invasive treatment. Therefore a situation occurs in which sufferers are not always seeking help and dental professionals may not necessarily be screening for the condition. This paper addresses the prevalence, etiology, diagnosis, and management of dentin hypersensitivity.
Introduction
To gain a greater insight into professional knowledge and attitudes around dentin hypersensitivity and the conditions which mainly predispose to it, a survey was conducted (Martin Akel & Associates, underwritten by a research grant).2 In its broadest strokes, the survey sought to determine trends and opinions relative to dentin hypersensitivity; a profile of patients who suffer from dentin hypersensitivity; and the etiology, diagnosis, and treatment options for dentin hypersensitivity.
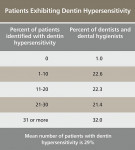
There were 1,704 usable responses (response rate = 8.8%) and there was a maximum sampling tolerance/margin of error of ± 2.4% at the 95% confidence level. Among the salient points, 75% or more of dentists and dental hygienists felt that the prevalence of gingival recession, erosion/tooth wear, and dentin hypersensitivity is increasing and that these conditions represent a challenge to long-term oral health and patient quality-of-life (Table 1). Approximately one third of respondents reported that over 30% of their adult patients suffered dentin hypersensitivity (Table 2), and there was little agreement as to the major cause (Table 3). Whereas these are only preliminary results, the full survey is expected to be published soon.
Furthermore, patients are not always routinely screened for dentin hypersensitivity, so it is under-diagnosed. Even when dentists and hygienists are aware that a patient has dentin hypersensitivity, they do not always take the time to discuss this condition with the patient—what is causing it and how to treat it—unless the patient asks.3 However, dentin hypersensitivity should be viewed as a patient complaint that needs to be addressed and managed as part of an overall treatment plan.
Dentin Hypersensitivity in the Context of Dental Pain
Dentin hypersensitivity is characterized by short, sharp pain arising from exposed dentin in response to stimuli—typically thermal (hot and cold), evaporative (air being blown or inhaled on a sensitive tooth), tactile (physical sensation when brushing or flossing a tooth), osmotic, or chemical—which cannot be attributed to other dental defects or pathology (Table 4).4 Gingival recession and tooth wear (especially erosion) both have causative links to dentin hypersensitivity and put the patient at risk for developing dentin hypersensitivity.5 While the term dentin hypersensitivity is preferable, it has also been referred to as tooth sensitivity, root sensitivity, or simply sensitivity. There is no doubt that early intervention in and patient education about gingival recession and erosive tooth wear are essential to preventing and treating dentin hypersensitivity and, in turn, avoiding restorative treatment.6
Oral or dental pain, on the other hand, may occur in and around the hard and soft tissues of the stomatognathic complex for a variety of reasons. For many people, the mouth can be very sensitive because of the presence of significant innervation of both the teeth and soft tissues. These nerve fibers are capable of identifying texture and shape to a minute level, temperature, and taste, and of course different types of pain. This rich innervation consequently identifies anything abnormal by increasing signals to the brain that may be interpreted as pain. Therefore, dental pain is that which arises from the teeth themselves or from the tissues immediately surrounding the teeth in direct response to a stimulus (eg, changes in temperature or pressure such as when eating, drinking, or tooth brushing) or as a result of the inflammatory process such as pulpitis due to a carious lesion or operative insult, or even as a result of maxillary sinusitis. Dental pain may be short and sharp, or longer in duration, dull and throbbing (Table 5).3 The pain may range in severity and frequency. When a diagnosis is elusive, consider referred pain or pain of neuropathic origin. In intractable cases referral may be necessary.
Among the patient complaints of dental pain is tooth sensitivity. Tooth sensitivity occurs in many different dental conditions (eg, pulpitis, cracked/chipped teeth, failing restorations, etc),7 but it is most commonly caused by dentin hypersensitivity. Dentin hypersensitivity is one of the most common causes of tooth sensitivity.8
The most common sites for dentin hypersensitivity are the buccal-cervical sites on the canines and premolars. Root sensitivity is most commonly a result of gingival recession. Dentin hypersensitivity in the coronal aspect of the tooth may be caused by loss of enamel resulting from erosive tooth wear, attrition, or acute trauma (tooth fracture).
Differential Diagnosis and Predisposing Conditions
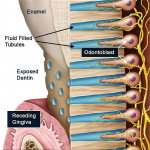
Because a number of other conditions exhibit similar signs and symptoms, the diagnosis of dentin hypersensitivity is a diagnosis of exclusion.9 When a patient experiences dentin hypersensitivity, two processes must have occurred: (1) the dentin must have been exposed (a process of lesion localization); and (2) the dentin tubule must be open throughout its full length from the oral cavity to the pulp. Opening of a normally occluded dentin tubule on the tooth surface, usually through loss of smear layer, is the process of lesion initiation (Figure 1). Under normal conditions, dentin is not exposed because it is covered either by the periodontal tissues or with enamel. For dentin to be exposed there is a loss of gingival tissues through recession or a loss of enamel (through tooth wear or trauma), except in cases of anatomical defects such as palato-gingival grooves or where the enamel margin falls short of the cementum.
The mechanism of gingival recession is not well understood but overly aggressive oral hygiene or neglected oral hygiene and inflammation due to plaque accumulation are major factors. A primary predisposing factor for dentin hypersensitivity is gingival recession with subsequent exposure of root surfaces. Gingival recession most frequently occurs on the facial surfaces. It has been reported that 88% of people ages 65 years and older and 50% of those between the ages of 18 and 64 have one or more sites with recession, and the presence and extent of gingival recession increases with age.10 These sites are at risk to dentin hypersensitivity and thus warrant screening for dentin hypersensitivity.
The impact of enamel loss and tooth wear on the occurrence of dentin hypersensitivity cannot be underestimated. Erosion, abrasion, and attrition seldom occur in isolation,11 and it is generally understood that erosion and abrasion most often occur simultaneously. Various acids are common in the 21st century diet, and it is accepted that an increase in acid intake corresponds with an increase in erosion.12,13 Generally a pH of 5.5 or lower is capable of softening the surface
3 µm to 5 µm of enamel within only a few minutes (Table 6).3 If left undisturbed rehardening may occur over a period of hours. Enamel, while in this softened state, is vulnerable to physical abrasive forces leading to irreplaceable loss and thus tooth wear. Once enamel is lost, the exposed dentin is subject to wear and chemical erosion resulting from acid attack. Dentin may be demineralized at pH levels as high as 6.5 and remineralizes very poorly.3
Tooth sensitivity with symptoms identical to dentin hypersensitivity may often arise consequent to dental procedures. Patients who have undergone periodontal therapy, for example, are approximately two to three times more likely to experience postoperative dentin hypersensitivity than the general population. In fact, an estimated 73% to 98% of periodontal patients reported dentin hypersensitivity compared to approximately 36% in the general population.14 During periodontal therapy, dentin tubules commonly become exposed and a lesion of dentin hypersensitivity initiated. This condition is often self-limiting, as in the weeks after therapy the tubules become occluded and symptoms subside. Likewise, following placement of a restoration, polymerization shrinkage may occur, yielding symptoms of sensitivity, usually of a transient nature. Also, sensitivity is one of the most commonly reported complaints among users of in-office or at-home dental bleaching materials.15 Clinical studies investigating bleaching sensitivity report that between 55% and 77% of patients who used tray-based bleaching products experienced sensitivity.15 While sensitivity during whitening has been reported to be transient the symptoms are often severe and for many patients it is a barrier to continue treatment. Though it is unlikely that the aforementioned episodes of sensitivity are strictly dentin hypersensitivity, these factors need to be considered in arriving at a differential diagnosis.
Not all exposed dentin results in the symptoms of dentin hypersensitivity. Even when dentin is exposed it is usually covered by a smear layer made up of loosely bound organic and calcified debris that clogs the dentin tubules so that they are not open to the oral cavity. When the smear layer is removed, the tubule openings become exposed and are then susceptible to external stimuli, which can lead to dentin hypersensitivity.17 The smear layer is easily removed by dietary acids, gastric acids, and also detergents found in toothpastes and rinses (Figure 2).18,19
A Common Problem?
Prevalence of dentin hypersensitivity varies from 4% to 57%, with a figure of 15% fairly consistently reported. The permanent canines and premolars are the teeth most frequently affected, and the sites on those teeth most commonly affected are the buccal cervical regions. Symptoms can present at any age, but the majority of individuals range from ages 20 to 40, with a peak in prevalence at the end of the third decade. Women are more frequently affected, and at a younger mean age, possibly due to more frequent and extensive oral hygiene episodes. The decline in prevalence after age 40 may be due to the levels of secondary and reparative dentin. There are also reports of discrepant perceptions of prevalence, with hygienists reporting twice the level of dentin hypersensitivity in patients as dentists.20
Reports in the dental literature list factors contributing to an increased prevalence of dentin hypersensitivity, and many clinicians report an apparent earlier age of onset, even in the teens and early twenties. Whereas dentin hypersensitivity traditionally has been regarded as a condition of older patients with receding gingivae, lifestyle changes in the current diet, such as frequent and increasing ingestion of highly acidic foods and beverages (eg, fresh fruits and salads, fruit juices and carbonated beverages), that contribute to increased rates of erosive tooth wear may help explain this earlier onset.
Management of Predisposing Conditions
With the definitive diagnosis of dentin hypersensitivity, a treatment plan can be formulated and recommendations can be made to the patient. The overall plan should address the causes and the symptoms so that when treatment has been initiated it will have the greatest chance of success.21
Gingival Recession
The patient with gingival recession and dentin hypersensitivity can be educated to minimize future problems. The patient should be informed and taught to adopt less-aggressive oral hygiene habits and consider changing from a manual toothbrushing technique to a power toothbrush with soft bristles. The patient should be encouraged to change any parafunctional oral habits that are contributing to recession. For a smoker, the dentist or dental hygienist can make recommendations for smoking cessation. As part of the initial clinical examination, any defective restorations or anatomic abnormalities such as frenas that are contributing to recession can be addressed with restoration replacement or surgical correction.
Tooth Wear
Tooth surface loss can be categorized as loss of enamel or dentin. Enamel can be lost by tooth wear or by trauma. Tooth wear is caused by physical loss (abrasion, attrition, and/or abfraction), chemical loss (erosion), or most commonly a co-effect of chemical and then physical activity (Table 7).5,22,23
Abrasion is usually caused by excessive toothbrushing with highly abrasive toothpastes and/or aggressive oral hygiene habits (hard bristles with much manual force, reloading the brush with paste, etc).
Attrition is wear resulting from tooth-to-tooth contact such as occlusal parafunctional habits, or in the regular function of mastication of abrasive foods.
Abfraction is a non-carious cervical lesion (NCCL) hypothetically caused by occlusal parafunctional forces. Loss of enamel resulting from trauma can lead to exposed dentin. Abfraction also puts the tooth at risk of dentin/root surface chemical erosion.
Chemical erosion of the enamel and dentin is most commonly produced by excessive and frequent intake of an acidic diet and less commonly by the reflux of hydrochloric acid from the stomach. Acids demineralize and soften the enamel and dentin surfaces, making them more susceptible to abrasion, particularly by toothbrushing with or without toothpaste.24,25
Multifactorial causes include the combination of erosion and abrasion, with erosion initiating wear and abrasion localizing it to a particular part of the tooth.4,26
The fact remains: once enamel is lost, the underlying dentin is exposed and in most cases requires a restorative procedure.9,27,28 Failure to restore the tooth and exposed dentin often leads to dentin hypersensitivity. In the case of the exposed dentin on the root surface, the depth and width of the NCCL helps dictate the need for restorative intervention. In some cases, the exposed root surface of a NCCL can lead to root caries.
The diagnosis and treatment of erosion requires a comprehensive medical and dietary history. Many times erosion can be managed and prevented through behavior modification to decrease the frequency and severity of exposure.28 Also, it is important to identify the role of salivary flow to dilute and flush acids from the oral cavity and increase remineralization. For some patients, medications may contribute to a decreased salivary flow. As part of treatment recommendations to enhance enamel remineralization, the teeth can be treated with in-office fluoride agents, prescription fluorides for home use, toothpastes specifically formulated for erosive-prone patients which optimize fluoride uptake while minimizing abrasivity, and use of a correct (soft and gentle) brushing technique.29,30 For the patient already at risk because of enamel and dentin erosion, recommendations can be made to the patient to wait several hours before toothbrushing after acid food and beverage exposure, to avoid frequent intakes of acidic beverages, to avoid swishing of acids around the mouth, and to consume foods rich in calcium (eg, yogurt, milk, cheese) after acid exposure.31
Following Periodontal Therapy
Managing dentin hypersensitivity in patients who have undergone periodontal therapy has involved recommending the use of a potassium nitrate desensitizing toothpaste twice daily in cases of slight to moderate hypersensitivity. For those patients with prolonged or severe hypersensitivity, there may be the need for an in-office treatment recommendation to support the desensitizing dentifrice at home. This can include the use of fluoride varnishes;32 applications of sealants and bonding agents and placement of restorations;33,34 having the roots surgically covered with gingival grafts;9 and in the most extreme cases, when no other treatment has been successful and the patient can no longer tolerate the sensitivity, endodontic treatment of the offending tooth to leave the tooth non-vital and insensitive.
Following Tooth-Whitening Therapy
Bleaching-induced tooth sensitivity is thought to occur because enamel is permeable and, as such, allows the active bleaching agents (eg, hydrogen and carbamide peroxide) to enter the tooth, where it diffuses through the dentin and reaches the pulp.3 There it can either directly stimulate the pulp, making it sensitive, or release minute bubbles of oxygen inside the tooth—particularly inside the dentin tubules—which creates pressure. This form of transient sensitivity subsides upon cessation of bleaching.
The management and/or treatment of bleaching-induced sensitivity has involved the use of a potassium nitrate-containing desensitizing toothpaste twice daily for 2 weeks before, as well as during, the bleaching regimen; reduced frequency, duration, and concentration of the bleaching regimen; or discontinued bleaching in severe cases of hyper-
sensitivity.35 A practice-based clinical study involving over 200 patients in 14 practices investigated the efficacy of desensitizing toothpastes to relieve sensitivity related to bleaching. Patients using the desensitizing toothpaste experienced many more days free of sensitivity than did patients using a regular toothpaste, and were more satisfied with the overall tooth-whitening experience.35
Treatment of Dentin Hypersensitivity
The first step toward early intervention of dentin hypersensitivity is screening and diagnosis. Additional vigilance is required for any patient exhibiting gingival recession or erosive tooth wear. Once diagnosed the patient should be counseled on preventable risk factors for the recession or tooth wear as appropriate, provided with instructions for relief of symptoms through everyday use of desensitizing toothpaste, possibly provided with application of an in-office desensitizer, and then followed up after 2 to 3 weeks to determine if the symptoms have been relieved, remained the same, or become more severe. In the latter instances the differential diagnosis should be reviewed, and if still confirmed as dentin hypersensitivity, the clinician should review oral hygiene practices and confirm the use of the desensitizing toothpaste in accordance with manufacturer’s instructions, and consider further in-office treatment. At times some patients will continue use of their previous toothpaste as well. Commonly this may be a whitening or tartar control formula whose more powerful detergents may continue to be removing the smear layer and opening more tubules. It is particularly important for the desensitizing toothpaste use to continue once symptoms have initially subsided because many patients suffering from dentin hypersensitivity will experience recurrent pain and treatment relapse (Figure 2). In some cases, patients will follow at-home treatment recommendations at first, but will not continue treatment once the symptoms have been relieved, only to have the symptoms return.
Patients value recommendations from their dentist and hygienist. It is important that clinicians making recommendations for the use of a desensitizing dentifrice include how to use the toothpaste. For most patients using a potassium nitrate desensitizing toothpaste, reduction in dentin hypersensitivity will take at least 2 to 3 weeks with continued use to avoid a relapse of symptoms. The treatment effect builds over time with the maximum relief beginning to occur after 8 to 12 weeks of twice-daily use. Therefore, it is important for good patient compliance with this recommendation to change from their regular toothpaste to a desensitizing toothpaste that the patient is able to select a desensitizing toothpaste with similar characteristics to their former paste (total care, gum care, breath freshening, whitening, gel, etc).
In contrast to patient-applied desensitizing agents, professionally applied desensitizing agents are often limited to specific sites. The most commonly used in-office desensitizing agents are high-concentration fluoride, metallic salts, varnishes, sealants, and bonding agents.
Fluoride treatments contain a high concentration of fluoride and are generally prescription strength. Fluoride treatments may be applied to many sites. Fluoride varnishes, used for the remineralization of tooth enamel and prevention of caries, reduce the sensitivity for a short time, but abrasion of the varnish tends to make the effect short-lived. Often, multiple applications are necessary. The implication is that the benefit comes from the physical blockage of the dentinal tubules by the varnish base rather than the fluoride.
Fluoride is thought to work by a reaction between the fluoride ion and ionized calcium in the tubular fluid to form an insoluble calcium fluoride precipitate. While there is good evidence that applications of concentrated fluoride have a beneficial short-term effect on sensitivity, the fluoride concentration in toothpastes and rinses purchased over-the-counter do not seem to be high enough to have an impact.3
Metallic salts, commonly oxalate, are applied in a variety of ways, generally as solutions. These salts are often effective desensitizers and they work by occluding the dentinal tubules. These are available as aluminum, potassium, or ferric oxalates.3
Varnishes are commonly used to deliver fluoride. There are various types of varnishes and some will be used with the intent of blocking the tubules while others will be used to deliver higher concentrations of fluoride. Additionally, light-cured and self-curing bonding and adhesive resins used in the placement of restorations have produced encouraging results when applied over sensitive dentin to seal off dentinal tubules, preventing tubular fluid movement. This technique is useful when there is persistent, severe sensitivity that is very clearly localized. A thin topical veneer protectively covers exposed tubules to temporarily block pain stimuli. Over time, with brushing and normal function, the varnish may eventually wear away and pain will recur.
Conclusion
Dentin hypersensitivity is a significant and prevalent issue facing dental practitioners. Two conditions—gingival recession and erosive tooth wear—most commonly predispose a patient to suffer the symptoms of dentin hypersensitivity. Differential diagnosis is critically important, followed by a clinically appropriate management plan that also addresses any predisposing conditions. The currently recommended management/treatment continuum for persistent dentin hypersensitivity, depending upon the severity and extent of pain, should involve the least-invasive intervention. In some cases of long-term dentin hypersensitivity, gingival recession and tooth surface loss may involve more invasive periodontal and restorative treatments. In all cases with an initial early diagnosis, minimally invasive options (such as a change to desensitizing toothpaste) should be tried first. Considerations should be given to the use of in-office desensitizing agents to occlude patent dentin tubules36,37 or the use of fluoride varnishes. At-home recommendations of desensitizing toothpastes should be included as part of treatment recommendations. Planned follow-up to monitor treatment and reduction in pain also should be included as part of the recommendation.
Patients and their providers need to understand that the continued everyday use of anti-sensitivity or desensitizing toothpaste is a noninvasive, inexpensive, and effective first line of treatment for dentin hypersensitivity. Further, as part of the treatment recommendation, the toothpaste should have a desensitizing therapeutic agent and also have a formulation sufficiently low in abrasion to prevent other contributory conditions to dentin hypersensitivity, such as tooth wear as a result of erosion and abrasion, as well as be high in fluoride content to promote caries prevention and rehardening of acid-softened enamel.
Disclosure
This paper was commissioned by AEGIS Communications, and funded by GlaxoSmithKline.
References
1. Yip HK, Smales RJ, Kaidonis JA. Management of tooth tissue loss from erosion. Quintessence Int. 2002;33(7):516-520.
2. Dentin Hypersensitivity—Insights from Dental Professionals. Martin Akel & Associates; December 2007.
3. The Story of Dentin Hypersensitivity: Etiology, Diagnosis and Management. Module One. GlaxoSmithKline; August 2005.
4. Holland GR, Narhi MN, Addy M, et al. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808-813.
5. Bissada NF. Symptomatology and clinical features of hypersensitive teeth. Arch Oral Biol. 1994;39 Suppl:31S-32S.
6. Gandara BK, Truelove EL. Diagnosis and management of dental erosion. J Contemp Dent Pract. 1999;1(1):16-23.
7. Seltzer S, Boston D. Hypersensitivity and pain induced by operative procedures and the “cracked tooth” syndrome. Gen Dent. 1997;45(2):148-159.
8. Ngassapa D. Neurophysiological basis, aetiology and clinical aspects of hypersensitive teeth. East Afr Med J. 1996;73(12):775-778.
9. Jacobsen PL, Bruce G. Clinical dentin hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract. 2001;2(1):1-12.
10. Casaba MM, Cohen RE. The etiology and prevalence of gingival recession. J Am Dent Assoc. 2003;134(2):220-225.
11. Litonjua LA, Andreana S, Bush PJ, et al. Tooth wear: attrition, erosion, and abrasion. Quintessence Int. 2003;34(6):435-446.
12. Gleason P, Suitor C. Children’s diets in the mid-1990s: Dietary intake and its relationship with school meal participation. Alexandria, VA: US Department of Agriculture, Food and Nutrition Service, Office of Analysis, Nutrition and Evaluation; 2001.
13. Phelan J, Rees J. The erosive potential of some herbal teas. J Dent. 2003;31(4):241-246.
14. Drisko CH. Dentin hypersensitivity—dental hygiene and periodontal considerations. Int Dent J. 2002;52:385-393.
15. Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide tooth whitening (bleaching) products: review of adverse effects and safety issues. Br Dent J. 2006;200:371-376.
16. Jorgensen MG, Carroll WB. Incidence of tooth sensitivity after home whitening treatment. J Am Dent Assoc. 2002;133:1076-1082.
17. Addy M, Pearce N. Aetiological, predisposing and environmental factors in dentine hypersensitivity. Arch Oral Biol. 1994;
39 Suppl:33S-38S.
18. Vanuspong W, Eisenburger M, Addy M. Cervical tooth wear and sensitivity: erosion, softening and rehardening of dentine; effects of pH, time and ultrasonication. J Clin Periodontol. 2002;
29(4):351-357.
19. West NX, Hughes JA, Addy M. Dentine hypersensitivity: the effects of brushing toothpaste on etched and undetached dentine in vitro. J Oral Rehab. 2002;29(2):167-174.
20. West NX. The dentine hypersensitivity patient: a total management package. Int Dent J. 2007;57(6 Suppl 1):411-419.
21. Addy M. Tooth brushing, tooth wear and dentine hypersensitivity
—are they associated? Int Dent J. 2005;55(4 Suppl 1):261-267.
22. Khan F, Young WG, Daley TJ. Dental erosion and bruxism. A tooth wear analysis from south east Queensland. Aust Dent J. 1998;43(2):117-127.
23. Khan F, Young WG, Shahabi S, et al. Dental cervical lesions associated with occlusal erosion and attrition. Aust Dent J. 1999;
44(3):176-186.
24. Narhi M, Yamamoto H, Ngassapa D, et al. The neurophysiologic basis and the role of inflammatory reactions in dentine hypersensitivity. Arch Oral Biol. 1994;39(Suppl):23S-30S.
25. Bartlett DW. The role of erosion in tooth wear: aetiology, prevention and management. Int Dent J. 2005;55(4 Suppl 1):277-284.
26. Eisenburger M, Shellis RP, Addy M. Comparative study of wear of enamel induced by alternating and simultaneous combinations of abrasion and erosion in vitro. Caries Res. 2003;37(6):450-455.
27. Barbour ME, Rees GD. The role of erosion, abrasion and attrition in tooth wear. J Clin Dent. 2006;17(4):88-93.
28. Yip KH, Smales RJ, Kaidonis JA. The diagnosis and control of extrinsic acid erosion of tooth substance. Gen Dent. 2003;51(4):
350-353.
29. Lussi A, Hellwig E. Risk assessment and preventive measures. Monogr Oral Sci. 2006;20:190-199.
30. Lussi A, Hellwig E, Zero D, et al. Erosive tooth wear: diagnosis, risk factors and prevention. Am J Dent. 2006:19(6):319-325.
31. Jaeggi T, Lussi A. Toothbrush abrasion of erosively altered enamel after intraoral exposure to saliva: an in situ study. Caries Res. 1999;33(6):455-461.
32. Ritter AV, de L Dias W, Miguez P, et al. Treating cervical dentin hypersensitivity with fluoride varnish: a randomized clinical study. J Am Dent Assoc. 2006;137:1013-1020.
33. Cardoso AC, Canabarro S, Myers SL. Dental erosion: diagnostic-based noninvasive treatment. Pract Periodontics Aesthet Dent. 2000;12(2):223-228.
34. Santamaria MP, Suaid FF, Nociti FH Jr, et al. Periodontal surgery and glass ionomer restoration in the treatment of gingival recession associated with a non-carious cervical lesion: report of three cases. J Periodontol. 2007;78:1146-1153.
35. Haywood VB, Cordero R, Wright K, et al. Brushing with a potassium nitrate dentifrice to reduce bleaching sensitivity. J Clin Dent. 2005;16(1):17-22.
36. Pereira JC, Segala AD, Gillam DG. Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre-treatments—an in vitro study. Dent Mater. 2005;21(2):129-138.
37. Azzopardi A, Bartlett DW, Watson TF, et al. The measurement and prevention of erosion and abrasion. J Dent. 2001;29(6):
395-400.
About the Authors
Howard E. Strassler, DMD
Professor and Director of Operative Dentistry
University of Maryland School of Dentistry
Baltimore, Maryland
Connie L. Drisko, DDS
Dean and Merritt Professor
Medical College of Georgia School of Dentistry
Augusta, Georgia
David C. Alexander, BDS, MSc, DDPH
Glaxosmithkline
Parsippany, NJ