Mechanisms of Resin Adhesion-Dentin and Enamel Bonding
Bart Van Meerbeek, DDS, PhD
Abstract
This article describes the mechanisms of adhesion to enamel and dentin as they are employed by today's dental adhesives, which pursue either an etch-and-rinse or a self-etch strategy. While micromechanical interlocking remains the primary adhesive mechanism, mild self-etch adhesives, in particular, may additionally make use of chemical interaction that especially contributes to the long-term stability of the bond. Although one-step adhesives are the simplest to use, their adhesive performance is less than that of multi-step adhesives, primarily due to lower bond strength and durability, phase-separation phenomena with hydroxy-ethyl-methacrylate (HEMA)-poor/free formulations, enhanced watersorption with HEMA-rich formulations, and a reduced shelf life.
The use of composite filling materials, along with adhesive techniques, has revolutionized today's dental practice. The esthetic potential, handling, and wear properties of composite fillings have improved tremendously.1 In the hands of a skilled dentist, today's composite fillings are able to replace lost tooth tissue without visible detection. However, no matter how splendid the shape and color, a satisfactory composite filling does not last long without a solid bond to the remaining tooth structure.
Basically, the main bonding mechanism of current resin adhesives can be regarded as an exchange process involving substitution of inorganic tooth material by resin monomers that, upon in situ setting, become micromechanically interlocked in the created microporosities.2 Diffusion is the primary mechanism to obtain such micromechanical retention. Recently, more evidence has corroborated the potentially important role of additional chemical interaction at the biomaterial-tooth interface, especially with regard to bond stability.3,4
Bonding Approaches to Overcome the Smear Layer "Obstacle"
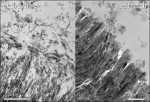
Cavity preparation alters the uppermost layer of tooth tissue, covering the tooth surface with a 1.0 µm to 2.0 µm layer of cutting debris (Figure 1). The orifices of the dentin tubules are obstructed by smear plugs contiguous with the smear layer consisting of shattered and crushed hydroxyapatite, as well as fragmented and denatured collagen. In clinical conditions, these may also be contaminated by bacteria and saliva. In order to overcome this smear layer obstacle, a certain degree of etching is required. Early nonacidic adhesives failed mainly because they did not penetrate deeply enough to establish a bond with the underlying intact dentin.
There are basically two options (ie, removal of the smear layer prior to bonding following an etch-and-rinse procedure, or the use of adhesives that can penetrate beyond the smear layer while incorporating it following a self-etch approach) (Table 1 and Figure 2).5 For both approaches, micromechanical interlocking is the basic mechanism of adhesion to enamel and dentin. Mild self-etch adhesives interact superficially with enamel/dentin, leaving sufficient hydroxyapatite available for additional chemical interaction, similar to the twofold bonding mechanism of glass-ionomers.5
The Etch-and-Rinse Approach
All categories of adhesives exhibit the common adhesion mechanism of hybridization. A hybrid layer results from the process of micromechanical interlocking ensuing a demineralization, infiltration, and polymer-setting process and was first described by Nakabayashi et al.6 Etch-and-rinse adhesives can readily be recognized by an initial etching or conditioning step, followed by a compulsory rinsing phase. They are often called "total-etch" adhesives. Note: this term is less appropriate because self-etch adhesives do etch tooth tissue, also, and are applied simultaneously to enamel and dentin. The etching step removes the smear layer/plugs, produces etch pits at enamel, and demineralizes dentin in order to achieve a microretentive surface. Originally, three-step etch-and-rinse systems typically consisted of three separate application steps (ie, conditioning, priming, and adhesive-resin application). In search of simplification, a two-step etch-and-rinse approach was developed to combine the priming and bonding steps into one; this approach is frequently referred to as "one-bottle adhesives," which misleadingly suggests a single-application step. Both three- and two-step etch-and-rinse adhesives pursue a similar adhesion mechanism.
Currently, bonding to enamel is still best accomplished through the use of the etch-and-rinse approach. Etching with 35% phosphoric acid removes the enamel top layer for a few micrometers and selectively dissolves hydroxyapatite crystals within prismatic and interprismatic enamel. This increases microscopic rough- ness, surface area, and energy. The simple application of a hydrophobic, ordinary bonding agent efficiently infiltrates the etch pits by capillary attraction. Upon in situ polymerization, micro- and macro-tags are formed that are responsible for the durable micromechanical interlocking achieved at enamel following this etch-and-rinse procedure (Figure 3, left). This bond to enamel not only effectively seals the restoration margin, but also protects the more vulnerable bond to dentin against degradation.7
At dentin, phosphoric acid removes the smear layer while concurrently demineralizing dentin over a depth of 3.0 µm to 5.0 µm, thereby exposing a scaffold of collagen fibrils nearly depleted of hydroxyapatite.8,9 The exposed collagen fibrils function as a microretentive network for micromechanical interlocking of resin monomers that diffuse into it and in situ polymerize, eventually forming a hybrid layer (Figure 4). Re-expansion of the collagen mesh that has collapsed upon postconditioning air drying is especially crucial to improve the bond strength of the adhesive. Neither the thickness of the hybrid layer or the length of the resin tags seems to play an important role regarding bond strength.10 Due to the inert nature of collagen fibrils and the low affinity of the monomers for hydroxyapatite-depleted collagen, true chemical adhesion between collagen and the methacrylate monomers is unlikely.2,5,9 Consequently, the poor adaptation and envelopment of resin to the collagen fibrils leave nanometer-size gaps that have been shown to absorb silver (Figure 5), representing the nanoleakage phenomena.11 This process is thought to be primarily responsible for the gradual degradation of the bond.12
Thus far, in vitro and in vivo research has indicated that etch-and-rinse adhesives can achieve high-quality adhesion to both enamel and dentin, and that three-step etch-and-rinse adhesives commonly exhibit superior performance over their two-step counterparts.13-15 The latter are also associated with greater technique sensitivity than the three-step adhesives; this is understandable since a single solution combines the two separate functions of primer and bonding resin. Moreover, after aging, the bonding integrity of three-step etch-and-rinse adhesives is better maintained,15 and they remain the gold standard among adhesives, despite the rather elaborate and lengthy working procedure required.
The primer solvent within etch-and-rinse adhesives is a major factor affecting their handling and performance. Water/ethanol-based adhesives are regarded as the most forgiving in terms of application errors, while acetone-based adhesives require the more challenging "wet-bonding" technique.16 New etch-and-rinse adhesive technology has consisted of the use of ter-butanol as solvent into a recent two-step etch-and-rinse adhesive.a This solvent has a vapor pressure similar to ethanol, but a better stability regarding adverse chemical reaction with monomers.
Also indicative of the renewed interest in the three-step etch-and-rinse approach are the introductions of a unidose application system with an etch-and-rinse adhesive,b a HEMA/TEG-DMA-free adhesive systemc (Figure 4), and the ethanol wet-bonding technique.17 This ethanol-based wet-bonding technique has been shown to result in a significantly better bonding effectiveness than the currently well-adopted, water-based wet-bonding technique. The former consists of the intermediate application of ethanol in order to enable hydrophobic resins to better infiltrate the by-phosphoric-acid-exposed collagen fibril network. Such hydrophobic resins are expected to reduce the interfacial watersorption potential, and thus, eventually improve the bond durability.
Self-etch Adhesives
Self-etch adhesives were first developed by raising the amount of acidic monomers in HEMA/water-based adhesives. They do not require a separate etch-and-rinse phase, as they contain acidic monomers that simultaneously condition and prime enamel and dentin. As a result, the dissolved smear layer and demineralization products are not rinsed away, but incorporated in the adhesive resin.5,18 Self-etch adhesives can be subdivided by the number of application steps (ie, a two-step version or simplified one-step "all-in-one" version) and by their acidity (pH) and resultant aggressiveness [ie, mild (pH≥2), intermediate (pH²1.5), and strong (pH≤1)] (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6).5,18 Mild self-etch adhesives demineralize dentin shallowly, forming a submicron hybrid layer, while leaving hydroxyapatite crystals around the collagen fibrils. Strong self-etch adhesives produce resin tags, along with a 3.0 µm to 5.0 µm thick hybrid layer resembling the interfacial interaction of an etch-and-rinse adhesive; all hydroxyapatite is dissolved within the hybrid layer. Intermediate self-etch adhesives exhibit morphological features that lie between the mild and strong self-etch adhesives.
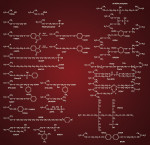
In spite of the small hybrid layer and the absence of resin tags (ie, minimal micromechanical retention), some mild self-etch adhesives do reach a satisfactory bond strength.5 Additional chemical interaction between the monomers and residual hydroxyapatite must explain the satisfactory performance of the mild self-etch adhesives (Figure 7).4,5 However, such interaction of self-etch adhesives with tooth substrate is dependent to a great extent on the kind of acidic functional monomer and eventually the overall composition of the adhesive. This generally results in a larger variation in bonding performance of the different self-etch adhesives currently available than of the etch-and-rinse adhesives, all of which rely largely on the use of phosphoric acid and the subsequent infiltration of monomers. The carboxylic and phosphate groups that render these monomers hydrophilic and that function as proton donors have been proven to bond ionically with calcium in hydroxyapatite.4 Figure 8 shows some poly-carboxyl and phosphate-based functional monomers with chemical bonding potential typically used in self-etch adhesives. The ability to chemically bond is monomer-specific and depends on the hydrolytic stability of the calcium-monomer bond. Using X-ray photoelectron microscopy (XPS), Yoshida et al demonstrated that 10-MDP exceeds the bonding potential of 4-MET and phenyl-P.4 The hydrolytic stability of the monomer itself is also important, particularly with regard to bond durability.3,12,19
When bonding to enamel, the strong self-etch adhesives generally perform better than the higher-pH self-etch adhesives. Nevertheless, with some mild self-etch adhesives,d a high and stable bond strength is achieved; this most likely must be attributed to the good chemical interaction potential of the functional monomer 10-MDP. Ultrastructurally, the interaction with enamel is quite shallow with the sole formation of microtags (Figure 3, right). While bonding to enamel may remain a problem, especially with mild self-etch adhesives, bonding to dentin has reached results comparable to those obtained by the gold standard three-step etch-and-rinse adhesives.5 Recent studies have also reported good clinical results for some self-etch adhesives.13,20
In comparison to etch-and-rinse adhesives, self-etch adhesives have had numerous advantages attributed to them. It has been suggested that they improve the efficiency in clinical procedures by omitting the obligatory rinsing phase in etch-and-rinse adhesives, thereby reducing chairside time. Conditioning, rinsing, and drying steps, which may be critical but difficult to standardize in clinical conditions, are eliminated in self-etch adhesives. Technique sensitivity associated with bonding to dehydrated demineralized dentin is eliminated, as rinsing and drying phases are no longer needed. Collapse of the collagen network is prevented, since monomers infiltrate concomitantly as they demineralize. Theoretically, incomplete resin infiltration is prevented in this manner as well. As the smear layer and smear plugs are not removed prior to the actual bonding procedure, rewetting of dentin by dentinal fluid from the dentin tubules is prevented, and a reduction of postoperative sensitivity has been reported.
The composition of self-etch adhesives is quite unique as they contain high concentrations of water and acidic monomers. Providing an ionization medium for the functional monomers, water is an indispensable ingredient of current self-etch systems.21 Two-step self-etch adhesives consist of a hydrophilic aqueous primer solution and a separate hydrophobic adhesive resin. Similar to the combined primer/adhesive resin solution of two-step etch-and-rinse adhesives, one-step self-etch adhesives are complex mixtures of hydrophilic and hydrophobic components. In particular, the high concentrations of water have raised questions about potentially harmful effects on polymerization, given that complete water removal is unrealistic.19 This also applies for the high concentrations of solvent, which may cause incomplete resin polymerization in instances of incomplete evaporation.
Current Concerns about One-step Adhesives
Although one-step adhesives (ie, 1-SEAs) are marketed as the most user-friendly, in vitro and in vivo research has revealed considerable shortcomings related to many of these simplified adhesives.
The relatively low bond strengths obtained by 1-SEAs are a main concern, especially when compared to multi-step self-etch and etch-and-rinse versions.2,5,14,15,22
Due to their high hydrophilicity, cured one-step self-etch adhesives have been demonstrated to act as permeable membranes, permitting water movement across the adhesive layer.23 Reticular patterns of nanoleakage (ie, water trees) have been found within the adhesive layer of 1-SEAs and are considered as sites of incomplete water removal and subsequent suboptimally polymerized resins.24 The actual clinical relevance of these water trees remains unclear, but since they may function as water ducts, they may contribute to accelerated degradation of tooth-resin bonds.12
All 1-SEAs are complex mixtures of monomers, solvents, and filler particles. These blends of hydrophilic and hydrophobic components have to simultaneously fulfill all the adhesive tasks of multi-step adhesives.5 This renders them sensitive to application errors. The air-drying step subsequent to their application is particularly crucial to reduce the amount of solvent and water in the adhesive layer as much as possible. It is known that thick adhesive layers of simplified adhesives decrease bond strengths tremendously.25 Conversely, this air-drying procedure should also be done in a manner that ensures that a sufficient amount of monomers is kept at the surface to provide satisfactory mechanical properties to the adhesive layer. In narrow and complex cavities, the correct balance between sufficient and insufficient air-drying is a precarious one; while the adhesive may still pool in the cavity corners, the amount of monomers on the cavity walls may already be too low.
More recently, complex processes of phase separation have been shown to occur in one-component, HEMA-free SEAs (Figure 9).26,27 As soon as such an adhesive is dispensed onto a glass plate and the solvent starts to evaporate, simple light microscopy of one-step adhesive solutions revealed a phase separation, yielding a multitude of droplets that slowly emerged toward the top of the adhesive drop to ultimately disappear. If the adhesive was light-cured, all remaining droplets were entrapped in the adhesive layer. The explanation for these observations must be found in the complex mixture of both hydrophobic and hydrophilic components, dissolved in an organic solvent (eg, ethanol or acetone). Gradual evaporation of the solvents sets off the phase-separation reaction, in which water separates from the other adhesive ingredients. HEMA plays a key role in this process, as this monomer acts as a wetting agent due to its hydrophilic character and can prevent water from separating from other adhesive ingredients. It is self-evident that the incorporation of droplets may contribute to bond degradation, but persistence of water in the adhesive layer may also adversely affect the bond strength. Nevertheless, these phase separations have been regarded as beneficial, as they also offer a way to remove water from the adhesive interface.26 Air-blowing of the primed surface will accelerate the evaporation of the solvent (eg, acetone), by which water separates from the other adhesive components. The resultant droplets can then be removed from the adhesive by a prolonged and thorough air blowing using maximum air pressure. In this manner, HEMA-free, one-step self-etch adhesives possess a type of built-in, relatively technique-insensitive method for achieving a low water-containing adhesive interface.
In order to avoid phase separation, HEMA can be added to the adhesive formulation, but a relatively high HEMA concentration is then required.27 However, such HEMA-rich adhesives are prone to other problems, in particular a higher sensitivity to water absorption from the outside environment and the host dentin. When HEMA is cured in the presence of water, polymerization is incomplete and a porous hydrogel is formed. These areas of increased permeability allow water to permeate through the adhesive layer, especially compromising the long-term bonding effectiveness.28 Another, but related, phenomenon is osmosis.27 The HEMA hydrogel produced upon curing of the all-in-one adhesive can act as a semipermeable membrane,23 in whose upper layer—insufficiently polymerized due to oxygen inhibition—is a hypertonic fluid. When both effects are combined, this results in an osmotic transfer of water from the underlying dentin toward the adhesive-composite interface. While, in principle, this is valid for all one-step self-etch adhesives, HEMA-rich adhesives are much more sensitive to this osmotic breakdown.27
As most resins used in dental adhesives are acrylates or methacrylates, the ester groups of these resins are prone to hydrolysis, particularly in an acidic environment.21,29,30 This degradation results in the formation of acids as methacrylic acid, enhancing the self-etching aggressiveness that is not necessarily accompanied by the needed deeper resin infiltration. Most of the commercially available adhesives are prone in some manner to such an in-the-bottle monomer degradation. One method to overcome this challenge is to produce water-free adhesives,f since such a monomer degradation is a hydrolytic process that will not take place in a water-free environment. To activate etching, such a water-free adhesive should be applied onto a wet surface; this immediately creates the problem of determining the wetness of the surface. Two-component one-step adhesives keep water separate from functional monomers.e,g,h,i,j,k More recently, synthesized monomers (eg, amide-linked polymerizable analogues) instead of conventional ester derivatives, and phosphonic-acid monomers instead of phosphoric-acid monomers (both in a light-cured, two-step, self-etch adhesivel) are more hydrolytically stable29 and, therefore, should improve the shelf life of the adhesives.
It has been shown that dental adhesives during the production of the hybrid layer also can release enzymes from the dentin matrix that exhibit low collagenolytic activity.31 Such host-derived enzymes can hydrolyze the collagen fibrils that make up the hybrid layer, and thus, compromise the effectiveness of the long-term clinical bonding. Recent enzymographic research has revealed that collagenase A is primarily responsible for the hydrolytic breakdown of the hybrid layer. The actual relevance of this enzymatic breakdown of the hybrid layer may, however, be less clinically dramatic than suggested in some papers, as the endogenic enzymes gradually decrease with age, generally disappearing before the age of 40.32 A way to protect the interface against such enzymatic degradation processes is through the use of matrix metalloproteinase-inhibitors, of which one, chlorhexidine, is already commonly employed in dentistry and seems effective in vitro, as well as in vivo.33
Conclusion
A proper classification of adhesives is indispensable for a good view of the current adhesives. The strength of the proposed classification lies in its simplicity and scientific basis. Each category in this classification is characterized by a specific bonding mechanism, a distinct application protocol, and a particular interfacial ultrastructure.
With regard to the actual bonding effectiveness, it is now quite clear that the in vitro and in vivo performance of an adhesive greatly depends on its specific ingredient composition; this is particularly true for the more recent one-step self-etch adhesives. In spite of the improved ease-of-use and faster application, a simplified application procedure so far seems to entail a reduced bonding effectiveness, and their advantages should therefore be traded off against their major shortcomings. Presently, the fairest tradeoff appears to be a mild two-step self-etch adhesive that combines micromechanical interlocking to the tooth substrate with additional chemical interaction that definitely contributes to the bond stability.
Acknowledgment
This article was written in part using data gathered from the adhesive dentistry research group of the Leuven BIOMAT Research Cluster, which consists of Eduardo Coutinho, Aline De Almeida Neves, Jan De Munck, Siegfried Jaecques, Paul Lambrechts, Atsuchi Mine, Selvi Palaniappan, Marleen Peumans, André Poitevin, Mouhamed Sarr, Kirsten Van Landuyt, Marcio Vivan Cardoso, and Francesca Zicari.
References
1. Summit JB. Fundamentals of Operative Dentistry: A Contemporary Approach. Chicago, IL: Quintessence Pub. Co.; 2006.
2. Van Meerbeek B, Vargas S, Inoue S, et al. Adhesives and cements to promote preservation dentistry. Oper Dent. 2001;Suppl. 6:119-144.
3. Inoue S, Koshiro K, Yoshida Y, et al. Hydrolytic stability of self-etch adhesives bonded to dentin. J Dent Res. 2005;84: 1160-1164.
4. Yoshida Y, Nagakane K, Fukuda R, et al. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83:454-458.
5. Van Meerbeek B, De Munck J, Yoshida Y, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215-235.
6. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265-273.
7. De Munck J, Van Meerbeek B, Yoshida Y, et al. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82:136-140.
8. Perdigão J, Lambrechts P, Van Meerbeek B, et al. The interaction of adhesive systems with human dentin. Am J Dent. 1996;9: 167-173.
9. Van Meerbeek B, Inokoshi S, Braem M, et al. Morphological aspects of the resin-dentin interdiffusion zone with different dentin adhesive systems. J Dent Res. 1992;71:1530-1540.
10. Yoshiyama M, Carvalho R, Sano H, et al. Interfacial morphology and strength of bonds made to superficial versus deep dentin. Am J Dent. 1995;8:297-302.
11. Sano H, Takatsu T, Ciucchi B, et al. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20:18-25.
12. De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118-132.
13. Peumans M, Kanumilli PV, De Munck J, et al. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864-881.
14. Inoue S, Vargas MA, Abe Y, et al. Microtensile bond strength of eleven contemporary adhesives to enamel. Am J Dent. 2003;16: 329-334.
15. Shirai K, De Munck J, Yoshida Y, et al. Effect of cavity configuration and aging on the bonding effectiveness of six adhesives to dentin. Dent Mater. 2005;21:110-124.
16. Tay FR, Gwinnett AJ, Pang KM, et al. Resin permeation into acid-conditioned, moist, and dry dentin: a paradigm using water-free adhesive primers. J Dent Res. 1996;75:1034-1044.
17. Nishitani Y, Yoshiyama M, Donnelly AM, et al. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85: 1016-1021.
18. Tay FR, Carvalho R, Sano H, et al. Effect of smear layers on the bonding of a self-etching primer to dentin. J Adhes Dent. 2000;2: 99-116.
19. Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003;69:726-731.
20. Peumans M, De Munck J, Van Landuyt K, et al. Five-year clinical effectiveness of a two-step self-etching adhesive. J Adhes Dent. 2007;9:7-10.
21. Van Landuyt KL, Snauwaert J, De Munck J, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28: 3757-3785.
22. Inoue S, Van Meerbeek B, Vargas M, et al. Adhesion mechanism of self-etching adhesives. In: Tagami J, Toledano M, Prati C, eds. Proceedings of 3rd International Kuraray Symposium on Advanced Adhesive Dentistry. Como: Grafiche Erredue; 131-148.
23. Tay FR, Pashley DH, Suh BI, et al. Single-step adhesives are permeable membranes. J Dent. 2002;30:371-382.
24. Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81: 472-476.
25. Zheng L, Pereira PN, Nakajima M, et al. Relationship between adhesive thickness and microtensile bond strength. Oper Dent. 2001;26:97-104.
26. Van Landuyt KL, De Munck J, Snauwaert J, et al. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84:183-188.
27. Van Landuyt KL, Snauwaert J, De Munck J, et al. Origin of interfacial droplets with one-step adhesives. J Dent Res. 2007;86: 739-744.
28. Tay FR, King NM, Chan KM, et al. How can nanoleakage occur in self-etching adhesive systems that demineralize and infiltrate simultaneously? J Adhes Dent. 2002;4:255-269.
29. Salz U, Zimmermann J, Zeuner F, et al. Hydrolytic stability of self-etching adhesive systems. J Adhes Dent. 2005;7: 107-116.
30. Nishiyama N, Tay FR, Fujita K, et al. Hydrolysis of functional monomers in a single-bottle self-etching primer-correlation of 13C NMR and TEM findings. J Dent Res. 2006;85:422-426.
31. Pashley DH, Tay FR, Yiu C, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83: 216-221.
32. Carrilho MR, Geraldeli S, Tay F, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86: 529-533.
33. Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Archs Oral Biol. 2000;45: 757-765.
a XP-Bond™, Dentsply International, York, PA
b OptiBond FL®, Kerr Corporation, Orange, CA
c cmf Adhesive System, Saremco Dental AG, Rebstein, Switzerland
d Clearfil™ SE Bond, Kuraray America, Inc., New York, NY
e Adper™ Prompt™ L-Pop™, 3M ESPE, St. Paul, MN
f Absolute2, Dentsply Sankin, Tokyo, Japan
g Futurabond NR, Voco America, Inc., Sunnyside, NY
h One-up® Bond F Plus, Tokuyama America, Inc., Encinitas, CA
i Reactmer Bond, Shofu Dental Corporation, San Marcos, CA
j Tyrian™ SPE, Bisco, Inc., Schaumburg, IL
k Xeno® III, Dentsply, York, PA
l AdheSE, Ivoclar Vivadent Inc., Amherst, NY
About the Author
Bart Van Meerbeek, DDS, PhD
Professor
Department of Conservative Dentistry (Leuven BIOMAT Research Cluster)
School of Dentistry
Oral Pathology and Maxillo-Facial Surgery
Catholic University of Leuven
Leuven, Belgium