Image Guidance for Implants Improves Accuracy and Predictability
An implant surgical guide or template is defined as a “laboratory-fabricated guide based on ideal prosthetic positioning of implants used during surgery.”1 The purpose for using a guide for implant planning and placement is to increase the accuracy and ability of restorative and surgical clinicians to provide precise, safe, and predictable outcomes for patients when implant dentistry is performed.
Surgical guides are created combining diagnostic anatomical information from a computerized tomographic scan combined with physical or virtual representations of the planned restorations. Today, most diagnostic scans are accomplished using cone beam computed tomography (CBCT) scanners. Office or laboratory fabricated radiopaque scanning appliances may be used to simulate the desired position of teeth, midline access holes, tissue deficits, etc.2 These dental restorative landmarks must be analyzed and compared to the patient’s underlying radiographic anatomy, enabling decisions about implant position, size, grafting requirements, and other parameters. When this computer-based planning technology is used to create surgical guides, the process becomes known as “computer-assisted manufacture surgical guidance.”3
Pretreatment Analysis
The overwhelming value of having this information available is so the dental technician, restorative dentist, and surgeon can anticipate the surgical obstacles and restorative outcomes before beginning treatment. More importantly, this pretreatment analysis creates an opportunity for negotiated compromise in implant positioning, restorative design, or patient expectations before treatment is performed. Termed “collaborative accountability” by Rosenfeld et al,2 this pretreatment outcome analysis reduces the possibility for poor results, misunderstandings, complications, and cost overruns.
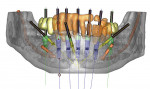
While the pretreatment analytic aspect of scanning guides is important, the digital data also provides the foundation for creating models and surgical guides and restorations from the same information. For this analysis, medical modeling of the data can be performed, converting it to 3-dimensional (3-D) virtual images. Rapid prototyping,4 3-D printing, or stereolithography1 are methods of fabricating accurate, solid, 3-D models from virtual data that can be used to replicate the patient’s bony anatomy. When interactive planning software is used, these manufacturing techniques can also be used to create surgical guides, directing drills to the positions of the planned implant osteotomies. Figure 1 shows an example of a sophisticated surgical plan that depicts bony topography, vital structures, ideal implant positions, guide stabilization pins, and the restorative design all on the same image.
Surgical guides can be fabricated to seat on teeth, soft tissue, or bone.5 Those that fit on bone frequently require significant flap elevation for surgical exposure of enough alveolar surface to create a precise stable fit. Bone-supported guides cannot be used for flapless surgery. It may be challenging to design this kind of guide when teeth are to be extracted in the surgical field. Data for these guides can be read from the diagnostic scan without additional diagnostic casts or intraoral imaging.
Tooth-supported guides require that an adequate number of stable teeth are available to support the guide. When teeth are relatively far from the anticipated surgical site, movement, distortion, or displacement of the guide can occur, reducing its accuracy. Tooth-supported guides may be used with either flapless or traditional surgical procedures.
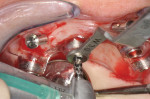
Tissue-supported guides seat on mucosal surfaces, potentially building in some inaccuracies due to the possibility for tissue compression or movement. Often these guides are designed to be stabilized using transverse locking screws or pins to prevent movement once the guide is inserted in the patient’s mouth (Figure 2).
There is currently no data to compare the accuracy of different manufacturers’ systems, or bone versus tooth, versus tissue-supported guides. In a systematic review, Jung et al6 noted a mean accuracy of 0.74 mm at the entry point and 0.85 mm at the apex for a variety of guides and systems. However, the range of error was clinically significant with maximum deviations reported of 4.5 mm at the entry point and 7.1 mm at the apex. Other accuracy studies7,8 noted similar results with various methods of template production, support, and stabilization.
Flapless Approach
Surgical guides are often recommended when flapless or minimally invasive procedures are performed since visible landmarks are obscured by tissue and structures. It is generally accepted that minimally invasive implant surgery has some potential advantages over traditional flap procedures,9 including less postoperative pain. Becker et al10 and Brodala11 speculated that the flapless approach may preserve periosteum, reduce bone resorption, and improve esthetics, particularly in thinner tissue. Barter12 utilized this concept to help preserve a reconstructed alveolar ridge previously grafted with an iliac bone graft by avoiding flap reflection.
Accurately performing flapless surgery without a surgical guide can be problematic. Van de Velde et al13 conducted an in vitro bench study using six dental students, six experienced restorative dentists, and six experienced periodontists using lifelike radiopaque models covered with artificial tissue. All participants were provided radiographic data, including reformatted CT scans with planning software and full ability to rotate the models on a bench. The participants were asked to perform flapless osteotomies on the models and duplicate the ideal implant positions determined from the planning software. Evaluation of the osteotomy positions after drilling indicated that all groups significantly malpositioned the osteotomies, even under ideal benchtop conditions.
Few statistically significant differences were seen between the groups in specific measurements, indicating that experience did not necessarily improve accuracy of osteotomy preparation. Further, they found that once the artificial mucosa was removed, nearly 60% of all osteotomies had dehiscence defects. In patients, the clinical implications of these dehiscence defects could include recession, inflammation, or bone loss—clearly unintended consequences of inaccurate flapless surgery. Although not performed in this study, it would seem that incorporating accurate surgical guides prepared with respect to the underlying anatomy would improve outcomes, preventing malposition and dehiscences.
Surgical guides, whether CAD/CAM or laboratory-fabricated, have some drawbacks. They have some bulk, which may interfere with surgical procedures. They could impede flap reflection and surgical visualization. Computer-generated guides have metal sleeves embedded within them, which closely match the diameter of the drills to be inserted through them. Irrigation cooling of the drills could be impeded, leading to overheating bone and healing complications. Additionally, the sleeves typically require the use of relatively long drills, creating potential inter-arch space problems.14
Updates, Improvements, Future Applications
For both tooth- and tissue-supported guides there is a requirement that the patient’s oral anatomy (teeth and/or alveolar contours) be merged with the virtually planned osteotomy positions. This can be done by sending the patient’s casts to the guide manufacturer, scanning a cast with a CBCT, or combining data from an intraoral optical scanner. This information is then merged with the CBCT digital imaging and communications in medicine (DICOM) data, creating a composite of radiographic and topographical (oral hard and soft tissue) data to be used for virtual planning, guide, or even restoration fabrication. With proper planning and rapid prototyping manufacturing software, a virtual wax-up, implant position, abutment design, surgical guide, provisional restoration, and final restoration could all be planned and fabricated digitally. Even alveolectomy, alveoplasty, implant position, and provisional restorations can be anticipated, guided, and planned with these techniques.15
Prefabricating restorations using digital planning, guided surgery, and rapid prototyping or milling is possible but has not become routine due to various difficulties and limitations. Several authors16-19 describe different methods and systems to create these restorations. While preoperative analysis, planning, and preparations for these respective systems are complex and time-consuming, they appear to provide accurate, minimally invasive, and predictable results.
Prerequisite to prefabricating immediate restorations is the ability to precisely control implant depth and rotational orientation from planning through surgical placement. Surgical guides produced with rapid prototyping techniques can be designed either for drilling the osteotomies (partially guided) or additionally for placing the implants (totally guided).20 An increasing number of systems offer the option of total guidance, which also requires special surgical instruments for implant placement.
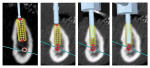
Another technology being developed for implant planning and surgery is surgical navigation.21 Used in orthopedics, neurosurgery, and ENT surgery, this technology uses visual or tactile feedback to communicate the desired implant position to the surgeon rather than a rigid guide (Figure 3). The computer is “connected” to the patient and the drill, and displays or directs the drill in real-time relative to the planned position. Elian et al22 confirmed the accuracy of one system, which compared favorably to surgical guides. He further noted that there may have been potential advantages of navigation since there was no appliance to interfere with drill cooling and drill lengths could be shorter so as not to collide with opposing dentition. Additionally, since the patient’s radiographic anatomy was displayed during surgery in real-time, instantaneous modifications in the plan could be made during the procedure with full anatomical information.
Workflow, Cost, Practice Integration
As with any new technology, a learning curve is associated with implementing image guidance into practice. Patients to be treated with image guidance require a computerized scan, most likely CBCT. The data is exported into planning software where the dentist can analyze bony anatomy relative to the planned restoration, identify vital structures, and place virtual implants. Some planning software requires the user to convert the CBCT data into a proprietary format, termed reformatting, before analysis. Others directly use the DICOM files generated by the CBCT.
Analysis and planning of a case should become a collaborative process with the restorative dentist, surgeon, and patient. Decisions on implant number, position, angulation, grafting requirements, and surgical risk assessment can be made. While some radiology services offer implant planning as part of their service, the final responsibility for the accuracy and integrity of the plan rests with the treating dentists and cannot be delegated.
Surgical procedures are performed in the customary manner for dental implant placement. Additional instruments may be needed to step down the diameter of the guide sleeves to match the smaller size drills. Each manufacturer sells sleeve sets to accommodate its guides and the specific implant drilling system. As previously mentioned, specific attention should be paid to ensure the guide is accurately secured in position and that the drills are cooled during osteotomy preparation. During the procedure, the surgeon needs to verify that the position of the osteotomy is appropriate and that an error did not occur in analysis, planning, or guide manufacturing. This requires that the surgeon possess enough surgical knowledge and skill to evaluate and correct a potentially inconvenient or harmful mishap before damage occurs. After the osteotomy is prepared in a partially guided system or the implant is inserted in a fully guided system, the guide is removed. Additional procedures for patient treatment are then completed as necessary.
Conclusion
Mandelaris et al20 identified 10 factors that contribute to successful image-guided surgery, including quality of the imaging, reformatting, radiographic scatter, and rapid prototype medical modeling. Radiographic interpretation of thin crestal bone and distortion of the stone casts used for topographic imaging can affect the outcome. Finally, surgical guide fit, stability, clinician skill, and experience affect results.
Incorporating image guidance into implant practice offers many advantages for the treatment team as well as patients. The greatest value is that preoperative planning rather than intraoperative mental navigation drives treatment. This can give the treatment team adequate time and accurate, intuitive tools for case planning to achieve superior, consistent results. Compromises, modifications, alterations, and cost considerations can be evaluated, discussed, and negotiated before initiating treatment. This reduces aggravation, complications, and misunderstandings. Future applications will facilitate faster, more comfortable, and more predictable implant dentistry, including more accurate diagnosis, minimally invasive surgery, immediate restoration/loading, and reduced treatment time.
CT-based image guidance cannot be considered a substitute for adequate training, sound clinical judgment, experience, or expertise. Technology cannot usurp biology, no matter how sophisticated these systems become.
References
1. Laney WR, ed. Glossary of Oral and Maxillofacial Implants. Chicago, IL. Quintessence Publishing; 2007.
2. Rosenfeld AL, Mandelaris GA, Tardieu PB. Prosthetically directed implant placement using computer software to ensure precise placement and predictable prosthetic outcomes. Part 1: diagnostics, imaging, and collaborative accountability. Int J Periodontics Restorative Dent. 2006;26(3):215-221.
3. Kopp KC, Koslow AH, Abdo OS. Predictable implant placement with a diagnostic/surgical template and advanced radiographic imaging. J Prosthet Dent. 2003;89(6):611-615.
4. Popat AH. Rapid prototyping and medical modeling. Phidias Newsletter. Denmark; 1998;1:10-12
5. Mandelaris GA, Rosenfeld AL. Surgiguide Options. In: Tardieu PB, Rosenfeld AL, eds. The Art of Computer-Guided Implantology. Chicago, IL: Quintessence Publishing; 2009:67-88.
6. Jung RE, Schneider D, Ganeles J, et al. Computer technology applications in implant dentistry: a systematic review. Int J Oral Maxillofac Implants. 2009;24(suppl):92-109.
7. Schneider D, Marquardt P, Zwahlen M, Jung RE. A systematic review on the accuracy and the clinical outcome of computer-guided template-based implant dentistry. Clin Oral Implants Res. 2009;20(suppl 4):73-86.
8. Valente F, Schiroli G, Sbrenna A. Accuracy of computer-aided oral implant surgery: a clinical and radiographic study. Int J Oral Maxillofac Implants. 2009;24(2):234-242.
9. Fortin T, Bosson JL, Isidori M, Blanchet E. Effect of flapless surgery on pain experienced in implant placement using an image-guided system. Int J Oral Maxillofac Implants. 2006;21(2):298-304.
10. Becker W, Goldstein M, Becker BE, Sennerby L. Minimally invasive flapless implant surgery: a prospective multicenter study. Clin Implant Dent Relat Res. 2005;7(suppl 1):S21-S27.
11. Brodala N. Flapless surgery and its effect on dental implant outcomes. Int J Oral Maxillofac Implants. 2009;24(suppl):118-125.
12. Barter S. Computer-aided implant placement in the reconstruction of a severely resorbed maxilla—a 5-year clinical study. Int J Periodontics Restorative Dent. 2010;30(6):627-637.
13. Van de Velde T, Glor F, De Bruyn H. A model study on flapless implant placement by clinicians with a different experience level in implant surgery. Clin Oral Implants Res. 2008;19(1):66-72.
14. Ganeles J, Grossberg D. Complications related to immediately loaded dental implants. In: Froum SJ, ed. Dental Implant Complications: Etiology, Prevention, and Treatment. Ames, Iowa: Wiley-Blackwell; 2010;356-377.
15. Balshi SF, Wolfinger GJ, Balshi TJ. A protocol for immediate placement of a prefabricated screw-retained provisional prosthesis using computed tomography and guided surgery and incorporating planned alveoplasty. Int J Periodontics Restorative Dent. 2011;31(1):49-55.
16. Rosenfeld AL, Mandelaris GA, Tardieu PB. Prosthetically directed implant placement using computer software to ensure precise placement and predictable prosthetic outcomes. Part 3: stereolithographic drilling guides that do not require bone exposure and the immediate delivery of teeth. Int J Periodontics Restorative Dent. 2006;26(5):493-499.
17. Sanna AM, Molly L, van Steenberghe D. Immediately loaded CAD-CAM manufactured fixed complete dentures using flapless implant placement procedures: a cohort study of consecutive patients. J Prosthet Dent. 2007;97(6):331-339.
18. Tahmaseb A, De Clerck R, Wismeijer D. Computer-guided implant placement: 3D planning software, fixed intraoral reference points, and CAD/CAM technology. A case report. Int J Oral Maxillofac Implants. 2009;24(3):541-546.
19. Tahmaseb A, De Clerck RD, Eckert S, Wismeijer D. Reference-based digital concept to restore partially edentulous patients following an immediate loading protocol: a pilot study. Int J Oral Maxillofac Implants. 2011;26(4):707-717.
20. Mandelaris GA, Rosenbeld AL, King SD, Nevins ML. Computer-guided implant dentistry for precise implant placement: combining specialized stereolithographically generated drilling guides and surgical implant instrumentation. Int J Periodontics Restorative Dent. 2010;30(3):275-281.
21. Casap N, Tarazi E, Wexler A, et al. Intraoperative computerized navigation for flapless implant surgery and immediate loading in the edentulous mandible. Int J Oral Maxillofac Implants. 2005;20(1):92-98.
22. Elian N, Jalbout ZN, Classi AJ, et al. Precision of flapless implant placement using real-time surgical navigation: a case series. Int J Oral Maxillofac Implants. 2008;23(6):1123-1127.
About the Authors
Jeffrey Ganeles, DMD
Assistant Clinical Professor, Periodontics
Nova Southeastern University
Ft. Lauderdale, Florida
Private Practice
Boca Raton, Florida
George A. Mandelaris, DDS, MS
Private Practice
Periodontics and Dental Implant Surgery
Park Ridge and Oak Brook Terrace, Illinois
Alan L. Rosenfeld, DDS
Clinical Professor
Graduate Periodontics
University of Illinois, College of Dentistry
Chicago, Illinois
Private Practice
Periodontics and Dental Implant Surgery
Park Ridge and Oak Brook Terrace, Illinois
Louis F. Rose, DDS, MD
Clinical Professor of Periodontics
University of Pennsylvania School of Dental Medicine
Philadelphia, Pennsylvania
Private Practice
Philadelphia, Pennsylvania