Use of a Sugar-Crosslinked Collagen Membrane in Conjunction With a Dehydrated Amnion/Chorion Membrane for Guided Bone Regeneration Around Immediate Implants
Matthew J. Fien, DDS; Israel Puterman, DMD, MSD; Juan Mesquida, DDS; Ferran Llansana Fitó, DDS; and Guillermo Bauza, PhD
ABSTRACT
Several bioabsorbable membranes have been proposed to exclude soft-tissue ingrowth and to stabilize the bone graft when guided bone regeneration (GBR) is performed. The properties of the various membranes differ slightly due to variances in composition and manufacturing processes affecting their handling and suitability for specific techniques. The aim of this article is to present a technique to perform GBR with the use of a sugar-crosslinked absorbable collagen membrane in conjunction with a dehydrated amnion/chorion membrane (dHACM). This technique can be used to perform GBR at the time of tooth extraction and for ridge augmentation of an edentulous site in preparation for future dental implant placement. The use of a collagen membrane in combination with a dHACM can facilitate stabilization of the bone graft and membrane by providing the benefits of a long-lasting bioabsorbable collagen membrane in addition to the unique benefits of an amnion/chorion membrane, which has been shown to provide growth factors to the surgical site that have potential to enhance regenerative outcomes.
Various materials and techniques can be used to achieve regeneration of a deficient alveolar ridge in preparation for implant placement.1-3 The use of a bioabsorbable collagen membrane has been successfully demonstrated by acting as a cell-occlusive barrier to prevent soft-tissue ingrowth into a particulate bone graft and by confining the bone graft material to the defect, aiding in stabilization of the graft-membrane complex.4 Several limitations exist to the use of a bioabsorbable collagen membrane for GBR related to specific handling characteristics, inherent memory, and early absorption with potential loss of graft volume if oral exposure is not avoided.4 The use of a dehydrated amnion/chorion membrane (dHACM) has been proposed to avoid such complications. dHACM has been investigated in the medical and dental literature, providing benefits of improved handling with the addition of biologic growth factors to the surgical site.5 Derived from the amniotic sac allografts and processed to retain amniotic membrane composition and bioactivity, dHACM contains over 250 biologic factors, extracellular matrix proteins, cytokines, interleukins, and tissue inhibitors of metalloproteinase, known to play a role in wound healing and reduce inflammation.6,7
This case report illustrates the use of a sugar-crosslinked collagen membrane in conjunction with dHACM to facilitate graft stabilization. This approach may simplify the GBR procedure and improve treatment outcomes due to inherent biological and structural properties of dHACM.
Technique (Materials and Methods)
This proposed procedure is completed in a manner similar to previously described GBR techniques utilizing an absorbable collagen membrane.4 In summary, access to the defect is achieved by placing incisions to bone followed by full-thickness mucoperiosteal flap reflection. The surgical site must be free of all granulomatous tissue and adequate bleeding at the site can be achieved by the placement of intra marrow decortication into the medullary bone. Tension release of the flaps is then completed as described by Ronda and Stacchi in 2015.8 A bone allograft can then be adapted to the ridge defect with or without the addition of autogenous bone scrapings. Combining allografts with autogenous bone has been encouraged to increase the quality and quantity of bone regeneration.9
A sugar-crosslinked absorbable collagen membrane (Ossix® Plus, Datum Dental Ltd, datumdental.com) is trimmed and adapted over the bone graft material. At this point, a dHACM (BioXclude®, Snoasis Medical, snoasismedical.com) is applied over the graft/collagen membrane using a dry instrument such as a tissue forceps. It is important that the instrument is as dry as possible to prevent premature hydration of the dHACM, which makes adaptation more difficult and time-consuming. Intraoral hydration of the dHACM is completed by holding the membrane in the desired position with the dry tissue forceps and making contact with the membrane with an instrument such as a periosteal elevator dipped in sterile saline. Gentle hydration of the entire membrane is completed by stroking the wet instrument over the entirety of the dHACM membrane such that the membrane loses all memory and sucks down over the particulate graft materials and overlying bioabsorbable collagen membrane. At this point stabilization sutures can be used if desired to improve stabilization of the graft and to increase the quantity of graft material that can be adapted to the defect.10
Primary closure of the surgical site is then achieved by placing flap-everting horizontal mattress sutures with a non-absorbable suture material such as polytetrafluoroethylene (PTFE) followed by multiple single interrupted, continuous interlocking or continuous double interlocking sutures. Healing of 4 to 6 months is required for sufficient turnover of the bone graft materials depending on the size of the defect being augmented.11
The following cases illustrate this technique of using a collagen membrane in conjunction with a dHACM for GBR.
Case Reports
Case 1
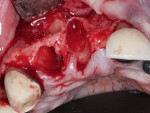
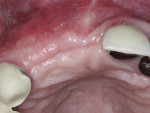
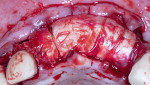
A healthy 65-year-old man presented with a hopeless prognosis of the maxillary right lateral and central incisors. After a comprehensive intraoral and radiographic examination, the patient was scheduled for extraction and immediate implant placement, with concurrent bone grafting of the site. Following sufficient local anaesthesia, periotomes were used to perform atraumatic extraction without the elevation of a mucoperiosteal flap. All granulomatous tissue was then debrided from the sockets, and bone sounding was completed, which revealed a 3 mm diameter fenestration of the buccal plate at the apex of the maxillary lateral incisor as well as a 11 mm to 12 mm deep dehiscence of the buccal plate at the maxillary central incisor (Figure 1). Due to the extent of the buccal plate defects, a full-thickness mucoperiosteal flap was elevated to provide access to the depth of the defects for access and subsequent placement of regenerative materials.
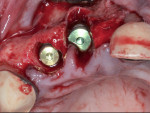
After confirmation that all granulomatous tissue had been debrided from the sockets, the presence of sufficient palatal bone was noted, allowing for adequate primary stability of the immediate implant. Implant placement was completed at this time following the manufacturer's recommendations (BioHorizons Tapered Internal, BioHorizons, biohorizons.com). Cover screws (0 mm height) were then placed in the implants and tightened to approximately 10 Ncm (Figure 2).
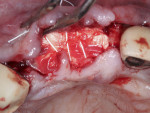
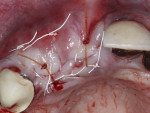
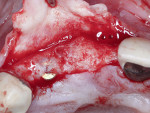
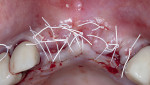
Following implant placement, a freeze-dried bone allograft (MinerOss®, BioHorizons) was used to graft the residual sockets surrounding the implants as well as the buccal plate defects that were present. A crosslinked collagen membrane (25 mm x 30 mm) was trimmed and hydrated in sterile saline for 90 seconds. The membrane was then adapted over the bone graft material with care taken to have the membrane extend apically 2 mm to 3 mm past the margin of the defects and over the buccal occlusal palatal line angle. Also, 1 mm to 2 mm was maintained between the membrane and adjacent teeth. Then, a dHACM was placed over the collagen membrane as described earlier to ensure confinement of the graft materials to the defect (Figure 3). A 1 mm deep periosteal releasing incision was then made along the entire length of the incision line located just apical to the mucogingival junction to release tension in the flap in preparation for closure. Two horizontal mattress sutures were then placed with a 3-0 PTFE suture to reduce tension in the flap margins and to initiate primary closure. Primary closure was then completed with subsequent single interrupted sutures with 3-0 PTFE as well as 4-0 chromic gut sutures (Figure 4).
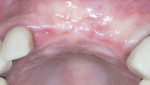
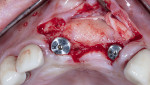
Healing at 2 weeks revealed no signs of infection. However, a 2 mm to 3 mm gap was noted between the buccal and palatal flaps at this time. Within this gap, immature gingiva could be seen granulating in over the exposed area. Healing at 5 months following treatment revealed a healthy site with adequate ridge width (Figure 5). Implant exposure was then completed to replace the cover screws with healing abutments. A horizontal incision was placed in the edentulous span, 2 mm to 3 mm palatal to the mid-crest. A full-thickness mucoperiosteal flap was elevated and excellent healing and turnover of the bone graft material was observed (Figure 6). The implant at the maxillary lateral incisor site was partially covered with mature hard tissue, while the implant at the central incisor site was completely covered with new hard tissue. No remnants of the particulate graft material could be identified. A back-action chisel and a surgical handpiece with a diamond bur were carefully used to completely expose the cover screws of the implants, and healing abutments were placed in preparation for subsequent implant crown fabrication several weeks later.
Case 2
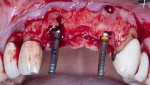
A 60-year-old male patient presented with a hopeless maxillary right central incisor, edentulous maxillary left central incisor, and hopeless maxillary left lateral incisor. Because minimal tooth structure was available for extraction of the maxillary left central incisor, a sulcular incision was placed extending from the maxillary right canine to the maxillary left canine, and a full-thickness flap was elevated. Extraction was completed followed by degranulation of the sockets. A 5 mm deep buccal dehiscence was noted at the site of tooth No. 8. A 3 mm buccal plate dehiscence as well as a 3 mm diameter buccal plate fenestration were observed at the apex of tooth No. 10. Adequate bone to support immediate implant placement was noted, and implantation (BioHorizons Tapered Internal) was completed at sites Nos. 8 and 9, followed by the placement of 0 mm height cover screws (Figure 7 and Figure 8).
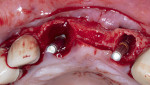
Adequate bleeding bone was observed; therefore, intramarrow decortications were deemed unnecessary in this case. A freeze-dried bone allograft composed of a 50/50 mix of cortico-cancellous graft material was then adapted to the sockets and buccal plate of sites Nos. 8 through 10. A sugar-crosslinked collagen membrane (25 mm x 30 mm) was then hydrated and adapted over the buccal occlusal palatal line angle. Due to the size of the edentulous span and addition of bone graft materials, the membrane was unable to fully cover the bone graft, and 2 mm to 3 mm of graft material was exposed on the mesial and distal aspects. A 20 mm x 30 mm dHACM was placed over the surgical site and extended past the defect margins mesially and distally 3 mm to 4 mm in both directions. Two periosteal biting stabilizing sutures were then placed with a resorbable suture to stabilize the collagen and amnion/chorion membranes over the graft material (Figure 9). Two horizontal mattress sutures with 3-0 PTFE were placed 5 mm to 7 mm from the soft-tissue margins to initiate tension-free primary closure. Additional single interrupted sutures using the same 3-0 PTFE were also utilized to complete primary closure (Figure 10).
Healing at 5 months revealed no signs of infection and significant maintenance of the anatomical ridge dimensions (Figure 11). A scalloped, horizontal crestal incision was completed followed by full-thickness flap reflection to expose the implants for placement of healing abutments. Upon flap reflection, complete maintenance of the ridge width and height was observed with no signs of the presence of any residual bone graft material. Implants were noted to be buried under 3 mm to 4 mm of regenerated hard tissue (Figure 12).
Discussion
Numerous collagen membranes are currently being used as a barrier for GBR procedures.11-13 One limitation to the use of a collagen membranes is an increased risk for loss of graft volume when the membrane is left exposed to the oral cavity or becomes exposed unintentionally during the early stages of healing.14 Interestingly, it has been shown that dHACM can be safely left exposed in the oral cavity. This is in part due to the inherent antibacterial properties that are beneficial to reduce the risk of infection following surgery.15 As a result, dHACM has been used as a membrane for a variety of oral surgical procedures such as GBR, guided tissue regeneration for periodontal intrabony defects, and socket preservation at the time of tooth extraction.16,17 Importantly, studies have illustrated similar new bone formation with the use of a collagen membrane versus the use of dHACM.18,19 The use of dHACM has also been shown to reduce postoperative pain following surgery.5
Due to the complete absence of memory following hydration of a dHACM, this membrane may be suitable for socket grafting and periodontal regeneration procedures, as it can be easily adapted over the alveolar ridge and around the arch for a long-span defect.18 In the authors' experience, dHACM has minimal inherent ability on its own to maintain the space required for predictable GBR of moderate and severe horizontal ridge defects as well as for vertical defects. On the other hand, it can be easily draped over the top of an absorbable collagen membrane to help stabilize the graft prior to final closure.
Conclusion
The use of a crosslinked collagen membrane in conjunction with a dHACM may be a viable option to improve treatment outcomes following GBR procedures. As shown in this case series, the use of the two different membranes provides the advantages of each of the particular membranes. The use of the crosslinked collagen membrane provides the necessary long-term barrier effect, some degree of memory that helps prevent collapse of the membrane into the defect, and the ability to ossify over time.20 The use of a dHACM offers great benefits as an adjunct to an absorbable collagen membrane, facilitating adaptation once a collagen membrane is in place over the bone graft due to its lack of memory. The growth factors and antibacterial properties of the amnion/chorion membrane may also provide substantial benefit over the use of a collagen membrane alone.7,15 The success of the cases provided in this study should be validated with an increased sample cohort. Therefore, further research on the use of the combination of a collagen membrane with adjunctive use of an amnion/chorion membrane is needed.
ABOUT THE AUTHORS
Matthew J. Fien, DDS
Private Practice Fort Lauderdale, Florida
Israel Puterman, DMD, MSD
Private Practice, Chevy Chase, Maryland
Juan Mesquida, DDS
Assistant Professor, Advanced Education in Implant Dentistry, School of Dentistry, Loma Linda University, Loma Linda, California
Ferran Llansana Fitó, DDS
Private Practice, Palma de Mallorca, Balearic Islands, Spain
Guillermo Bauza, PhD
Center for NanoHealth, Swansea University Medical School, Swansea, United Kingdom
REFERENCES
1. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22 suppl:49-70.
2. Urban IA, Jovanovic SA, Lozada JL. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: a retrospective study of 35 patients 12 to 72 months after loading. Int J Oral Maxillofac Implants. 2009;24(3):502-510.
3. Buser D, Dula K, Belser U, et al. Localized ridge augmentation using guided bone regeneration. 1. Surgical procedure in the maxilla. Int J Periodontics Restorative Dent. 1993;13(1):29-45.
4. Buser D. 20 Years of Guided Bone Regeneration in Implant Dentistry. 2nd ed. Hanover Park, IL: Quintessence Publishling; 2009.
5. Velez I, Parker WB, Siegel MA, Hernandez M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: a pilot study. J Periodontol. 2010;81(12):1797-1804.
6. Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10(5):493-500.
7. Lei J, Priddy LB, Lim JJ, et al. Identification of extracellular matrix components and biological factors in micronized dehydrated human amnion/chorion membrane. Adv Wound Care (New Rochelle). 2017;6(2):43-53.
8. Ronda M, Stacchi C. A novel approach for the coronal advancement of the buccal flap. Int J Periodontics Restorative Dent. 2015;35(6):795-801.
9. Sanders JJ, Sepe WW, Bowers GM, et al. Clinical evaluation of freeze‐dried bone allografts in periodontal osseous defects. Part III. Composite freeze‐dried bone allografts with and without autogenous bone grafts. J Periodontol. 1983;54(1):1-8.
10. Urban IA, Lozada JL, Wessing B, et al. Vertical bone grafting and periosteal vertical mattress suture for the fixation of resorbable membranes and stabilization of particulate grafts in horizontal guided bone regeneration to achieve more predictable results: a technical report. Int J Periodontics Restorative Dent. 2016;36(2):153-159.
11. Caballé-Serrano J, Munar-Frau A, Ortiz-Puigpelat O, et al. On the search of the ideal barrier membrane for guided bone regeneration. J Clin Exp Dent. 2018;10(5):e477-e483.
12. Sheikh Z, Qureshi J, Alshahrani AM, et al. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology. 2017;105(1):1-12.
13. Friedmann A, Gissel K, Soudan M, et al. Randomized controlled trial on lateral augmentation using two collagen membranes: morphometric results on mineralized tissue compound. J Clin Periodontol. 2011;38(7):677-685.
14. Eskan MA, Girouard ME, Morton D, Greenwell H. The effect of membrane exposure on lateral ridge augmentation: a case-controlled study. Int J Implant Dent. 2017;3(1):26.
15. Ashraf H, Font K, Powell C, Schurr M. Antimicrobial activity of an amnion-chorion membrane to oral microbes. Int J Dent. 2019;2019:1269534.
16. Holtzclaw DJ, Toscano NJ. Amnion-chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: a retrospective observational report. Clin Adv Periodontics. 2013;3(3):131-137.
17. Maksoud MA, Guze KA. Tissue expansion of dental extraction sockets using dehydrated human amnion/chorion membrane: case series. Clin Adv Periodontics. 2018;8(3):111-114.
18. Wallace S. Radiographic and histomorphometric analysis of amniotic allograft tissue in ridge preservation: a case report. J Implant Adv Clin Dent. 2010;2:49-55.
19. Koushaei S, Samandari MH, Razavi SM, et al. Histological comparison of new bone formation using amnion membrane graft versus resorbable collagen membrane: an animal study. J Oral Implantol. 2018;44(5):335-340.
20. Zubery Y, Nir E, Goldlust A. Ossification of a collagen membrane cross‐linked by sugar: a human case series. J Periodontol. 2008;79(6):1101-1107.