Efficacy of Preformed Sleep and Habit Appliances to Modify Symptoms of Sleep-Disordered Breathing and Oral Habits in Children With Focus on Resolution of Mouth Breathing
Earl O. Bergersen, DDS, MSD; Brooke Stevens-Green, BS, DDS; and Elizabeth Rosellini, DDS
ABSTRACT
Pediatric sleep-related breathing disorders (SRBDs) have been studied by various fields both diagnostically and therapeutically because of the detrimental symptoms associated with this condition in attempts to better understand the etiology, pathophysiology of associated comorbidities, and best means by which to screen, diagnose, and treat these patients. Common symptoms of SRBD or clinical conditions that can be exacerbated by sleep and airway issues include neurocognitive and behavioral problems, chronic inflammatory conditions, and skeletal and dental developmental issues. Method: This study of 220 children aged 2 to 13 years used a parental questionnaire to determine the initial severity as well as treatment changes over a mean 6.4 months of night-time passive wear of a removable preformed appliance. Objective: The study aimed to evaluate whether a preformed oral appliance could deliver clinical outcomes comparable to current therapeutics accepted in clinical practice for the treatment of pediatric SRBD (tonsil and adenoid tissue, myofunctional therapy, medication, other dental appliances); or in some instances, to discuss and compare the issues associated with each therapeutic pathway. Additionally, the research aimed to emphasize the need for a cross-disciplinary understanding and approach to the treatment of this pediatric condition to optimize clinical outcomes. Results: With passive night-time wear of the preformed appliance, 75% of total symptoms measured (N = 2054) experienced improvement, with a mean improvement of 76%. The most successful symptom corrected was headaches in the morning, where 85% of cases (n = 220) experienced complete correction (100%), while 98% of those having an improvement obtained a mean correction of 94%. The most frequently observed symptom was night-time mouth breathing, occurring in 69% of cases. Complete correction (100%) of night-time mouth breathing occurred in 43% of cases, while 83% of those cases with success had a mean improvement of 78%, when evaluating all symptoms measured (N = 2054). Conclusions: All 27 symptoms had statistically significant improvement, while 75% of total symptoms measured (N = 2054) experienced a mean improvement of 76%. Treatment changes were not dependent on differences in the patient’s gender, length of treatment time, the initial severity, and the age of the patient during treatment. 0.3% of all observed symptoms (N=2054) showed an increase in severity, and 24% had no change during the mean treatment period.
The intention of this retrospective cohort study is to provide sufficient evidence of the efficacy of preformed sleep and habit appliances to modify symptoms associated with sleep-disordered breathing (SDB) and oral habits in children. Additionally, a discussion of the efficacy of other popular therapeutics via the literature review initiates an argument to reconsider prioritization of these recommended interceptive therapies to optimize clinical outcomes. This research demonstrates the importance of studying these symptoms and therapeutics to determine the best means by which to improve the quality of life of these children, while also establishing a starting point for future prospective cohort analyses.
In recent decades, most North American children have been observed to present with at least one clinical symptom associated with a suboptimal sleep, airway, or breathing condition.1 Many of these symptoms present as confounding comorbidities, and a variety of therapeutic modalities have been suggested and/or supported from many disciplines, including but not limited to otorhinolaryngology, general pediatric care, myofunctional therapy (MFT), and dentistry. Each discipline has its own standard of care and prioritization of recommended care; however, there is insufficient research that demonstrates the benefit of a multidisciplinary approach and how to prioritize these different approaches.
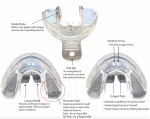
This retrospective cohort study aims to: (1) create a centralized, comprehensive literature review of the most up-to-date research related to pediatric SDB that evaluates both symptomology and therapeutics; (2) evaluate the frequency and degree of therapeutic efficacy of a removable preformed sleep and habit appliance (Figure 1) in modifying 27 symptoms associated with pediatric SDB; and (3) evaluate therapeutic efficacy related to other variables, including patient gender, total treatment time, and starting age of therapy.
Review of Literature
Diagnostic and Screening Tools Used
Various questionnaires to evaluate the prevalence and severity of SDB in children have been developed and vetted and are currently in clinical use. Most of these surveys are based on parental observation of the child's condition and behavior. The surveys are often used for an initial, baseline observation of the prevalence and severity of the symptoms associated with SDB as well as for ongoing measurement of the progression of these symptoms, regardless of whether there was therapeutic intervention or not.
The pediatric sleep questionnaire (PSQ) is a 22-item sleep-related breathing disorder (SRBD) questionnaire that was developed to help identify this pediatric condition via a less expensive and easier to use method relative to polysomnography2; this facilitates expanded clinical and epidemiological research. The PSQ was tested for validity and reliability and was confirmed to have a strong association with a diagnosis of SRBD (P < .0001) for children aged 2 to 18 years who had polysomnographically confirmed SRBDs.2 This diagnosis also demonstrated strong association with snoring (four items in the questionnaire, P < .0001), sleepiness (four items, P = .0003), and behavior (six items, P < .0001). The utility of this same PSQ was later studied for specificity and sensitivity when used in conjunction with pulse oximetry and clinical examination to evaluate children with obstructive sleep apnea (OSA)-hypopnea.3 It was concluded that with this questionnaire, clinical examination, and the addition of pulse oximetry screening, this was a sufficient diagnostic protocol for pediatric patients with suspected moderate to severe OSA-hypopnea.
Another questionnaire adapted from the Brouillette questionnaire4 by Villa5 tested 16 symptoms with a mandibular advancing appliance. This randomized controlled study used its questionnaire to evaluate the efficacy of a custom intraoral appliance to treat OSA in children with malocclusion. The oral appliance therapy was based on promoting the mandible in a downward and forward posture, stimulating the tongue for optimal function and positioning, and preventing and/or correcting any dental interferences that may predispose the child to have a compromised airway or breathing while sleeping. Significant treatment results were found for snoring, apneas, oral breathing, and nasal stuffiness. Those symptoms that did not reach significance were school success, nightmares, headaches upon waking, and increases in blood oxygen. These clinical results, as evaluated by the progression of observed symptoms via the questionnaire, demonstrated the custom oral appliance therapy to be "effective," as based on improvement of symptoms, and "well tolerated," as determined by a number of patients who completed the study as designed. However, the total patient count that completed the study was limited to 24 subjects.
Prevalent Symptoms Associated With Pediatric Sleep-Disordered Breathing
While a variety of diagnostic and screening modalities may exist to identify pediatric SDB, these tools share a similar pool of clinical variables associated with this condition. Most notable of these variables is the prevalence of mouth breathing, particularly mouth breathing while sleeping. Consistent through many diagnostic and therapeutic studies is the prioritization of addressing mouth breathing and the importance of establishing normal nasal breathing.6-8
Stevens et al indicated that night-time mouth breathing (NMB) is more prevalent than all of the 27 symptoms that were measured, occurring in 46% of the 501 cases of children observed aged 2 through 12 years.1 Self-correction did not occur in 93% of the entire sample, while 30% actually increased in severity from 4 through 12 years.
Mouth breathing during sleep is suspected of being caused by several factors. One main cause is allergic rhinitis, which tends to increase in occurrence as the child matures.9 With up to 40% of children affected, data suggests allergic rhinitis is the most common chronic disorder in the pediatric population.10 Because it often goes undiagnosed or untreated, many children develop multiple, related comorbidities, including sinusitis, asthma, conjunctivitis, eczema, eustachian tube dysfunction, and otitis media,11 all of which can contribute to decreased sleep quality.
Any factor that reduces normal nasal breathing, such as a deviated septum, congestion of the nose, and adenoid enlargement, can also have a major effect.12 In an analysis of 940 cases, it was found that 22.8% had difficult nasal breathing and 1.1% could not breathe through the nose at all, while 76% breathed normally without any nasal interference.13
NMB has also been linked to poor oral habits, including thumb sucking and extensive pacifier use.14 Thumb or finger sucking habits have been shown to create a vacuum-like condition within the oral cavity, which can be responsible for a narrowing or constriction of the upper posterior arch, abnormal tongue posture, labially positioned upper incisors, a retrognathic mandible, open bite, abnormal swallowing, and mouth breathing.14 These problems all have to be corrected or at least significantly improved to obtain major treatment success in reducing SDB, particularly NMB and snoring; these latter two symptoms are the major elements that must be resolved to obtain and maintain an open unrestricted airway.
Stevens et al found that 40% of the 10 most frequently occurring symptoms are directly associated with the practice of dentistry; these are daytime and night-time mouth breathing, bruxism, and snoring.1 There is increased awareness among dental providers to identify and treat these abnormal sleep issues as there appears to exist a positive biofeedback loop: disordered sleep, breathing, and other undesirable habits contribute to poor oral and facial development, which then in turn can exacerbate sleep and airway conditions while reinforcing the same bad habits.
Nocturnal enuresis (or "bedwetting") is a commonly observed behavior associated with SDB despite the unknown pathophysiological mechanism15; a large-scale community school-based survey of 20,000 primary school children (ages 5 to 12 years old) demonstrated that both SDB and unrefreshing sleep are potential independent risk factors of bedwetting in school-aged children.15
Alternative Therapeutics
Resolving mouth breathing is indicated as critically important to treat pediatric SDB (including specifically OSA6) in addition to supporting adequate craniofacial and airway development in children.7,8 Resolution of mouth breathing was also emphasized in being able to establish normal nasal breathing.6,16,17
The best means by which to eliminate mouth breathing and resolve other symptoms of SDB is a clinical matter of much debate; and "best" is difficult to define due to the complexity of this multifactorial problem that can be addressed by a variety of different healthcare providers (ear/nose/throat specialists [ENTs], dental providers, pediatricians, sleep physicians, myofunctional therapists, etc.) who yield a broad set of expertise and an even broader set of techniques to treat this condition.
Tonsil and adenoid removal-The otorhinolaryngology community has supported the correction of mouth breathing via removal of the swollen tonsillar and adenoidal tissue, although mouth breathing seems to persist after tonsil and adenoid (T and A) surgery in 55% of cases.7 Surgical removal of the tonsils and adenoidal swelling, often considered a successful treatment option, has resulted in rather conflicting evidence of success. Recent research indicates that this surgery corrects OSA in less than 25% of children treated and in only 10% of obese children.18-20 Significant results, however, have been reported by T and A surgery in asthma, snoring, blood oxygen saturation and apnea hypopnea index (AHI),21 hyperactivity, depression,22 and bed wetting.23
If T and A surgery is indicated in a child with a stage 3 or stage 4 tonsillar enlargement (according to the Friedman analysis),24 or if a child finds it difficult to or cannot breathe through the nose, a referral to a sleep clinic or ENT specialist is recommended prior to consideration of treatment with an oral appliance. Also, with the recommended use of a home monitor to aid in the diagnosis of OSA, any abnormality should be referred to a sleep clinic for further analysis.
Medications-This same otorhinolaryngology community along with pediatricians frequently utilize medications to decrease nasal obstructions in the hope of having the secondary effect of eliminating mouth breathing; many of these medications, however, induce undesirable side effects that cause children to be less compliant or even resist therapy. Over-the-counter remedies are associated with unnecessary costs, ineffective treatment, and/or undermedication leading to suboptimal control of symptoms.11 Oral histamines are typically a first line of defense, but the sedative and anti-cholinergic effects have been associated with decreased school performance.11 A more effective form of therapy includes intranasal corticosteroids, but long-term compliance can be deterred by the negative side effects that include dry nose, mucosal crusting, and bleeding.25
Oral appliance therapy-If a symptomatic child is either referred to or is first seen and screened by a dental professional, then oral appliance therapy is often utilized to address contributing factors to SDB. However, there are many different appliances, and their success can be dependent on even more variables, such as appliance design and fabrication, doctor experience, patient compliance, and/or parent(s)' engagement in the recommended therapy. Rapid palatal expansion (RPE) is a popular treatment option for correction of SDB issues in children. Improvement is seen for OSA, AHI, total sleep time, and oxygen saturation.20 Stability of correction via RPE intervention alone was confirmed via a separate 12-month follow-up study.26 When RPE and T and A surgery are combined, an improvement of 80% was seen in OSA while AHI was resolved completely in 40% of cases.
The custom oral jaw-positioning appliance used in Villa et al's randomized controlled study showed diminished daytime and night-time symptoms in treated subjects but no change in the control group after 6 months of treatment; respiratory symptoms improved in all of the patients with complete resolution in 50% of the patients treated, while the control group was unchanged in these same variables.5 An interesting discussion point in this same study highlighted the unexplained finding that there was reduced tonsillar hypertrophy seen in many of the children after treatment with the oral appliance; it was theorized that this could potentially be associated with a reduced inflammatory reaction due to the patient shifting from mouth to nasal breathing, or that the tonsils appear small after the repositioning of the mandible in a downward and anterior posture.5
Another oral appliance used to correct abnormal sleepiness was a form of a monobloc.27 It resulted in lowering the AHI with no change in the level of blood oxygen.
Myofunctional therapy-MFT was also suggested as a means for improving treatment results for mouth breathing.20 Success with MFT, however, is dependent on the ability of the therapist as well as the motivation of the parent and patient. Cooperation for adults has been reported at 85%.27 Villa et al found improvement with MFT in lip closure (83%), AHI (72%), and mouth breathing (69%).28 Another MFT study showed OSA was reduced by 62%.29
Methodology
Subject Selection
The cases in the study (N = 220, Table 1) were children between ages 2 and 13 years; 98% of the total symptoms observed and studied, however, were associated with children between 4 and 12 years. All cases were reviewed by experienced dental professionals prior to treatment initiation.
These 220 pediatric cases were evaluated by several dental professionals across the country, all of whom were trained via the same continuing education advanced certification course approved by the Academy of General Dentistry's Program Approval for Continuing Education (PACE™). The coursework, taught by HealthyStart® by Ortho-Tain®, is comprised of a 2-day, in-person lecture and clinical training. As a result, these doctors use the same protocols in their offices to evaluate, treat, and manage the patients that fit within the criteria as described in this study.
That the subjects originated from different dental clinics with different overseeing dental providers was assumed not to be an issue because each doctor was trained using the same system, and the screening questionnaire (HealthyStart® Sleep and Speech Questionnaire, HealthyStart, thehealthystart.com) (Figure 2) and therapeutic device (HealthyStart® Habit Corrector® Appliance, Ortho-Tain, orthotain.com) (Figure 1) were the same regardless of location or overseeing doctor. The target number of subjects for enrollment was decided prior to the study to ensure a sufficient number with equal representation among patient gender and age.
Evaluated patients would qualify for therapeutic intervention based on meeting one of the following criteria: any child that had a single symptom of NMB or daytime oral breathing; any child that snores; any child suspected of possible OSA; any child with three or more symptoms was arbitrarily considered to be a candidate for treatment; any child with bedwetting (because this can be such a problem, this single symptom also qualified a child for treatment).
As related to subject management, the information obtained was recorded by the investigators in such a manner that the identity of the human subjects cannot readily be ascertained, directly or through identifiers linked to the subjects. Specific to this research, the sleep and breathing symptoms evaluated over the mean 6.4-month period included observational data of behavior as well as survey/interview procedure-based questionnaires.
No control group was selected, because the results of no therapeutic intervention were previously confirmed via Stevens et al using the same questionnaire as included in this study.1 Specifically, it was observed that self-correction of those symptoms did not occur in 93% of the entire sample (n = 501), while 30% actually increased in severity from ages 4 through 12 years. Additionally, the therapeutic intervention as used in this study is based on leveraging natural growth and development, so when a parent expresses interest in engaging their child in therapy, it could compromise the ultimate clinical outcome of a child if he or she were delayed a finite amount of time for the purposes of creating a control for this research.
Screening Tool and Data Collection
The sleep and speech questionnaire used for this study was developed specifically for dental professionals (Figure 2).30 The questionnaire has 27 of the most significant symptoms describing various abnormal breathing-related issues together with 11 speech questions. The questionnaire is based on several studies, namely Urschitz et al,31 Sahin et al,32 and others,33-35 and has been previously described by Bergersen.30 It consists mostly of those symptoms that are significantly (P = .001) related to various severities of snoring.
The inclusion of the speech questions is associated with a different hypothesis that these symptoms of abnormal speech could be correlated with myofunctional issues that may also contribute to SDB.
Each child's parent was instructed to observe the sleeping characteristics of their child over several nights in order to more accurately answer the questions in the survey. The severity of each symptom was categorized as: 0, not present; 1-2, mild; 3, moderate; 4-5, pronounced. The parent that initially completed the form at the start of treatment (T1) was requested to fill out the progress form (T2) at a mean of 6.4 months later.30
Therapeutic Tool
For interventional therapy, the doctor delivered to each child a preformed habit- and breathing-modifying appliance (HealthyStart Habit Corrector Appliance) (Figure 1), also known as a habit sleep appliance (HSA). Pending child age and extent of dental eruption, the child was given one of two sizes ("Kid" or "Preteen/Youth") and instructed to wear the appliance every night while sleeping. The preformed therapeutic appliance is specifically designed to yield the following therapeutic outcomes, but one of the specific aims of this study was to validate these hypothesized benefits from the indicated design features:
• correct the habits that contribute to breathing problems in children (such as eliminating thumb sucking)
• advance the lower jaw through repositioning and promoting optimal growth patterns (thus addressing mandibular retrognathism) while also preventing the lower jaw (and attached tongue) from displacing itself in a posterior direction during sleep
• improve tongue posture and function by preventing abnormal resting tongue posture and eliminating abnormal tongue thrust swallowing (which is associated with speech problems such as dropping consonants, hoarseness, and lisps)
• begin to address dental issues that may contribute to SDB, including: expand a narrow maxillary posterior arch width up to 3 mm by elevating the tongue into the palatal and reinforcing proper swallow with built-in palatal tabs to increase expansive forces; correct or close dental open bite; prevent any malocclusion interferences that may cause suboptimal positioning of the hard and soft tissue or suboptimal interarch relationships
The reason for and potential benefit of selecting a preformed appliance for the purposes of this study is that it eliminates biases such as custom design and fabrication of dental appliances, doctor-specific experience and/or preference with treatment, and differing compliance issues between non-uniform appliances. Also, the preformed appliance model facilitates more efficient appliance replacement in the case of appliance loss or damage, which can be a common occurrence among a pediatric patient population.
Specific to palatal expansion, studies have demonstrated improved OSA and SDB symptoms via RPE.20 This preformed HSA appliance cannot achieve the same amount of expansion that an RPE appliance can. However, it is presumed that some patients benefit from the 3 mm of expansion they can get with this preformed appliance to eliminate dental occlusal interferences that may negatively contribute to other habits or cause poor jaw relationships. Research by the present author (EOB) consisting of unpublished data, which included 1125 patients, determined that 22% of the patients had normal arch width, 73.2% were deficient ≤3 mm (and therefore could be treated sufficiently with the expansive forces of the HealthyStart appliance), 4.2% demonstrated narrowing of 4 mm to 6 mm (which would require an adjunctive appliance such as a Schwartz or quad helix), and approximately 0.5% were deficient ≥7 mm (which would require the force of a rapid palatal expander to achieve both orthodontic and orthopedic expansion necessary). In the 4.7% of cases where an adjunctive appliance would be required to achieve optimal expansion, these appliances could be worn in conjunction with the HSA to achieve the necessary therapeutic benefits of both appliances worn over the same period of time.
Statistical Analysis
The statistical results of this study are shown in Table 2. Statistical differences between males and females regarding their initial severity (T1) and between the percentages of treatment change (T1 = beginning of treatment, to T2 = end of treatment) all resulted in nonsignificance. Therefore, males and females were pooled for the various comparisons studied. Some gender variations for a few symptoms resulted (Table 3). Differences between variables used the t-test. The confidence interval (CI) was also used. Significance was considered at the P = .05 level.
Results
The information data that was considered most useful in analyzing the presence of abnormal sleep and possible treatment using the HSA procedure was separated into nine categories of symptom frequency, levels of improvement, and statistical results (Table 2). The most frequently observed symptom in the sample (N = 220) was NMB, which occurred 69% of the time (Table 2, column B, symptom No. 10). Based on the selection criteria and qualifications for therapeutic intervention detailed above, 96.8% of the sample of 220 cases would be candidates for treatment, and 3.2% would not be considered for appliance therapy.
From Table 2, it can be seen that every symptom had a statistically significant improvement between T1 and T2. The incidence of cases that demonstrated 100% correction of a specific symptom (Table 2, column G) varied from 85% (symptom No. 11, morning headaches) to 19% (symptoms Nos. 23 and 24, hard time listening and interrupts, fidgets with hands and can't sit quietly, respectively). The two symptoms indicating a possible OSA problem (symptoms Nos. 6 and 7, interrupted snoring/stoppage of breathing, Table 2, column G) had a 100% correction 70% of the time.
The most successful symptom corrected was headaches in the morning, where 85% of cases experienced complete correction (100%), while 98% of those having an improvement obtained a mean correction of 94%. The improvement in NMB had complete correction (100%) in 43% of cases, while 83% of those cases with success had a mean improvement of 78%. Further statistical findings indicated that successful treatment of NMB did not depend on the age of the patient (P = .001), length of treatment (P = .001), initial severity of the symptom (P = .001), or gender of the patient (P = .001).
Of all the symptoms measured (N = 2054), 0.3% of the observed symptoms had an increase in severity while 75% of the symptoms had a mean improvement of 76%, and 24% had no change during the mean treatment period using the appliance while sleeping.
Discussion
Over the past two decades a growing amount of research has materialized from various fields evaluating sleep and breathing disorders in children. This increased interest and focus on both diagnostics and therapeutics for this patient population is facilitating improved patient outcomes via more precise and/or easier-to-use screening and diagnostic tools, greater provider and parent awareness of these specific health concerns, enhanced therapeutic interventions, and other approaches that have allowed for broader access to high-quality, comprehensive care for children.
One of the main objectives of this research was to determine the potential therapeutic benefit of a preformed oral appliance to eliminate or greatly reduce poor oral habits and symptoms associated with sleep-disordered breathing. A modified version of previously developed patient surveys was used to identify children who demonstrate these common signs and symptoms associated with an obstructed airway. The theorized benefits of using a preformed appliance (and therefore the reason why the investigators were interested in evaluating this potential therapeutic intervention) are as follows:
• increase access to care via easy screening and easier-to-deliver care, especially for patients who are either not candidates or noncompliant with other diagnostic or therapeutic modalities (for example, parents who would prefer to explore a nonsurgical route first, or patients who have sensitive gag reflex and cannot tolerate a dental impression procedure to fabricate custom appliances)
• decrease user error or bias based on treating-doctor experience and/or clinical preference
• provide a less expensive alternative to custom, laboratory-fabricated dental sleep appliances
• provide a nonpharmacological, nonsurgical alternative for therapeutics as either a potential first-phase treatment modality or alternative when the patient experiences refractory symptoms
• create a solution that can be paired with other solutions, including but not limited to surgical procedures, medications, myofunctional exercises, or other dental appliances (for example, a rapid palatal expander that can be worn in conjunction with this preformed HSA)
• enable minimal risks and/or negative side effects relative to other potential therapeutic options (greatest risks include slight discomfort for the child upon initial wear or that the child will not use or wear the appliance at all, as opposed to the risks associated with anesthesia, surgery, medications, and/or the discomfort associated with an RPE device); the only equivalent therapeutic as related to risks and side effects would be the custom oral appliance,5 but even that would require the discomfort of the dental impressions procedure)
The benefits of using the appliance is supported by the observation of change of all symptoms measured (N = 2054) with 0.3% of the observed symptoms showing an increase in severity, 75% of the symptoms having a mean improvement of 76%, and 24% having no change during the mean treatment period using the appliance while sleeping. This would suggest that for those children who qualify for and are compliant with intraoral appliance therapy, there is a significant opportunity for improvement in many different symptoms, with the downside risk being experiencing no change in some symptoms (24% of all symptoms measured, N = 2054) and only 0.3% of symptoms worsening with care.
These findings could support that this preformed HSA is a good first line therapeutic due to ease of use and minimal risks and side effects. After a course of treatment, additional, more invasive therapeutics or those with increased risks or side effects could be deployed to achieve further resolution of whatever symptoms remain. It is understood that further analysis and discussion should be performed related to the 0.3% of symptoms that worsened with use of the HSA to better understand the mechanism of action and the risk/benefit analysis of continuing care if these symptoms are evaluated to be worsening earlier in treatment.
Other statistically significant treatment results indicated the following conclusions:
• There was significant (P = .001) improvement in all symptoms regarding the correction between T1 and T2. One exception was "bluish in color" (P = .01) with a sample size of only seven individuals.
• There was no difference (P = .001) in percentage of treatment improvement when compared to the length of time (months) of appliance wear.
• There was no difference (P = .001) in the percentage of treatment change when compared to the initial severity of the symptoms at T1.
• There was no difference (P = .001) in the percentage of treatment change when compared to the age when treatment was initiated.
• There was no difference (P = .001) between males and females in either the initial severity at T1 or in the percentage of treatment changes between T1 and T2.
• The mean percentage of cases receiving improvement was 75% while obtaining a mean improvement of 76% in those same cases.
• 38% of all cases obtained 100% correction while the mean correction of the entire sample (N = 220) was 59%.
Differences were tested using the initial symptom severity (T1) as well as the percentage change between T1 and T2, and these treatment changes were then compared to several variables. These variables consisted of: (a) length of treatment; (b) initial severity of the various symptoms; and (c) the treatment obtained at various ages. There were no significant differences between the initial symptom severities and the percentage of treatment changes from T1 to T2 with these three variables.
These conclusions indicate that it probably does not make much difference: (a) whether treatment occurs over a shorter or longer period of time (5 to 7 months of treatment versus ≥10 months of treatment); (b) if no difference in treatment results depending on the severity of the initial symptoms (1 to 2 level of initial severity at T1 versus 4 to 5 severity); or (c) regardless of the age the treatment was initiated (4 to 6 years versus 10 to 12 years of age). This third finding is unexpected in that it was previously hypothesized that treatment would be more successful if initiated earlier due to the assumption that poor oral habits would be less severe (due to less time to reinforce them), anatomical changes would be harder to make later in age due to decreased growth and development remaining, and symptoms would be more severe due to length of time the child has been experiencing them and potential for increased comorbidity development. This is an interesting finding that should be validated in follow-up studies.
Night-time Mouth Breathing
As seen in Table 2, NMB is the symptom with the highest incidence (69%, column B), which is in agreement with Stevens et al.1 It is important to recognize NMB as a major contributor to abnormal breathing issues, thus deserving a priority status.6,7,36 There are likely secondary factors present also, such as oxygen desaturaton.37
The genesis of mouth breathing whether during the day or night is probably due to several factors. In that mouth breathing can be at least partially eliminated with the use of an HSA or by MFT indicates that it is probably, at least partially, a repetition-type induced habit. Abnormal nasal breathing can be an initial cause of mouth breathing in an infant or child of any age. In a separate sample of 1008 children in an unpublished research study by the present author (EOB), those children with difficult or impossible nasal breathing had 44% more severity in the initial NMB than in those with normal nasal breathing. In fact, the children with difficult or impossible nasal breathing reported no NMB in only 18% and 22%, respectively, while normal breathing cohorts reported 34%. Daytime mouth breathing had similar conclusions, being 30% and 22% for difficult and impossible nasal breathing, respectively, while for normal nasal breathers it was 56% for no presence of NMB. This is evidence that nasal congestion can be a major factor in its possible adverse influence on mouth breathing.
Other important factors that might influence mouth breathing include anything that can result in abnormal tongue function or abnormal tongue resting posture and position, such as thumb or finger sucking, pacifier and nipple bottle feeding, and sleeping on the back,38-42 gravity,43 obesity,44 and enlarged tonsils and adenoid tissue.45-47
Habits that are present at birth or in utero are particularly detrimental to the proper development of the mandible and the maxillary arch. Of all such habits, thumb or finger sucking can be especially harmful, particularly during the first 2.5 years of life, at which time the upper and lower deciduous dentition have erupted into contact, which then tends to restrict any further changes in the antero-posterior relation between the upper and lower arches throughout development.
Since the final adult antero-posterior occlusion is established permanently by 20 months of age,48 strong unrestricted mandibular downward and forward growth during the first 2.5 years of life converts a severe newborn retrognathic mandible into a more normal relation with the maxilla (Figure 3).49 This initial growth and major adjustment occurs while there is no restricting deciduous inter-occluded dentition or overbite. This unrestricted growth brings the newborn's lower jaw into at least a partial proper alignment with the upper arch. When a newborn or fetus sucks their thumb, it restricts this free forward and downward mandibular growth adjustment prior to the established deciduous dentition, which can then produce a retrognathic mandible that will persist into adulthood with potential restriction of the oropharynx.49 The developed posterior occlusion and anterior overbite of the deciduous and permanent dentitions probably prevent future anterior advancement of the mandible beyond the maxilla in normally growing children. Such a posteriorly positioned mandible can often constrict the airway,50 producing breathing problems.
A prebirth thumb sucking habit that strongly persists after birth can inhibit this early and necessary anterior growth spurt of the mandible, which is about 8.7 mm for 1.3 years (6.5 mm per year).49 Other important results of eliminating digit sucking are the encouragement of natural palatal and nasal width development and the prevention of the development of a high-vaulted palate, provided the tongue establishes itself into the palate during resting posture with normal nasal breathing and also during swallowing and various speech sounds.
Other habits also can be detrimental to breathing such as abnormal resting tongue posture, abnormal swallowing and tongue thrust, and, of course, mouth breathing and an open bite. Infant thumb sucking, however, is possibly the most important influential habit, since it is closely associated with an early persistent retrognathic mandible, mouth breathing, and reduced palatal width development. Thumb sucking should be corrected as early as it is observed in a child, in combination with mandibular advancement, in order to gain a normal jaw relation by 2.5 years of age. Treatment should be initiated not only for abnormal sleep symptoms, but also in combination with associated habits to obtain the most advantageous result. The habit and sleep modifying appliance is a recommended treatment alternative, as it has been specifically designed for successful correction in an infant or a child of any age with sleep issues, various habits, and abnormal growth patterns. A study investigating the compliance level of such an appliance worn while sleeping resulted in 93.4% cooperation.51
Headaches
Morning headaches was the most effectively treated observed symptom with night-time wear of the HSA. It is presumed that this is likely due to improvement and/or resolution of multiple factors, including improved oxygen saturation during night-time, decreased bruxism, and/or overall improved sleep quality/more restorative sleep patterns. Further analysis is recommended to better understand this correlation.
Limitations of This Research
The authors acknowledge that inclusion of a control group would have enhanced this study. It was not intended in the initial design, as this was a retrospective cohort analysis of existing non-identifiable patient data to evaluate larger patterns and highlight any hurdles or concerns for future studies associated with these same diagnostic and therapeutic tools. Also, the retrospective nature of this study explains why institutional review board (IRB) approval was not obtained prior to analysis and writing. Ideally, the findings of this initial study will trigger interest in more robust follow-up studies.
This analysis did not account for evaluation of tonsil and adenoid tissue (and/or history of previous T and A surgery), presence and/or severity of a tongue tie, or history of medications associated with SDB symptoms (both over-the-counter and prescribed).
Future Needs and Opportunities for Related Research
In further studies it would be essential to analyze either by polysomnography or a reputable home study initially at both T1 and T2 to verify the presence or absence of OSA, oxygen desaturation, reliable objective data on snoring, bruxism, nasal breathing and mouth breathing data, and obesity. The presentation of parental-answered questionnaires represents what is obtained in dental offices and is representative of current obtainable information of the parent, dental professional, and patient. At the present time, the dental profession is being encouraged to incorporate a home study analysis that can accurately monitor at least eight to 10 variables regarding sleep issues for children.
Currently, one study (with target N = 100) is in the preliminary stages of IRB approval that will incorporate this same diagnostic survey to calibrate its findings relative to CBCT, dental clinical examination, and polysomnography to include the supervisory expertise of dental providers, otolaryngologists, and head and neck radiologists.
The most valuable study would involve larger-scale therapeutic research to evaluate clinical outcomes of children who elect different treatment pathways (eg, tonsils and adenoid surgical removal, medication, custom oral appliance, RPE, no therapeutic intervention, MFT, or preformed HSA). Made even stronger would be an analysis of these pathways over a shorter, more acute period of treatment (6 months), as well as longer-term stability analyses at 12 months, 5 years and/or 10 years. The longer-term stability analysis would be critical in that many therapeutics have been studied in a 12-month follow-up, but very few have been re-evaluated at the completion of growth and development.
While this would be optimal for comparative learning, there are many issues with this approach as related to feasibility:
• Therapy decisions are not influenced solely by clinical criteria and doctor recommendations, but often are influenced by insurance reimbursement/cost (especially because some dental and alternative therapies are not covered or parents lack coverage).
• This research strategy would require the cross-disciplinary coordination between many healthcare providers who work in different locations and have very different diagnostic criteria to determine recommended treatment.
• It is assumed that the only variable that would be divergent would include the chosen therapy, but realistically, each child presents with a complexity of issues effecting anatomy, age, growth, environment, habits, parental engagement, health education, etc.; so this would require a very large number of subjects to ensure sufficient patient population to evaluate for potential conflicts or biases.
• Many of the symptoms associated with SDB can drastically decrease the quality of life of a child, and many children often experience a combination of therapies to achieve an optimal clinical outcome. It would be unrealistic and not in the best interest of the patient to expect that they would be limited in therapeutic choices if the therapy failed or if they experienced refractory symptoms or increased comorbidities. Additionally, many of these proposed therapeutics are most effective when delivered at the earliest age possible, so delaying until completion of a research study could negate an optimal clinical outcome for some patients.
While the aforementioned prospective cohort analyses are in various stages of planning and execution in collaboration with additional clinical and research teams, the investigators of this study plan to proceed with specific studies as follows: a next-phase study to evaluate the clinical outcomes of patients who do not elect to proceed with recommended therapy to assess for any potential placebo effect; a similar study framework to evaluate the clinical outcomes of pediatric patients with special needs treated with the preformed HSA; and a scientific review and more individual analysis from this set of data of the following subset of symptoms: speech issues, ADD/ADHD, and throat infections and allergies.
Lastly, reports from providers and further review of literature have indicated interest in testing hypotheses that would evaluate the prevalence of these SDB symptoms relative to other patient health history variables not evaluated in this study or included in other patient surveys, including breastfed versus bottle-fed (as this can influence skeletal development, habits, and systemic health), nutrition, presence and/or severity of a tongue tie, history of medication use, history of chronic otitis media, and patient compliance/parent engagement factors.
Conclusion
All 27 symptoms had statistically significant improvement, while 75% of total symptoms measured (N=2054), experienced a mean improvement of 76%. Treatment changes were not dependent on differences in the patient’s gender, length of treatment time, the initial severity, and the age of the patient during treatment. 0.3% of all observed symptoms (N=2054) showed an increase in severity, and 24% had no change during the mean treatment period.
DISCLOSURE
Dr. Bergersen is the innovator of the HSA. He retired from clinical orthodontic practice approximately 20 years ago to focus on research and development. Dr. Bergersen sits as an advisor on the board of the company that manufactures and distributes the HSA, but he volunteers his time and takes no compensation for this role. While neither Drs. Bergersen nor Stevens-Green have commercial interest in the product or the company that manufactures and distributes the HSA, they both share a family relationship with the owner and CEO of the company. Dr. Rosellini has no commercial interest nor family relationship with the other investigators or anyone at the company that manufactures and distributes the HSA. She is an independent dental provider that has used the product in her private practice for more than 10 years and trains other doctors on how to use the system in their clinics.
ABOUT THE AUTHORS
Earl O. Bergersen, DDS, MSD
Former Assistant Professor for 25 years, Northwestern University Dental School, Graduate Orthodontic Department, Chicago, Illinois; Formerly in Private Practice in Orthodontics, Winnetka, Illinois
Brooke Stevens-Green, BS, DDS
Private Practice, Pontiac, Michigan
Elizabeth Rosellini, DDS
Private Practice, Dorado, Puerto Rico
REFERENCES
1. Stevens B, Bergersen EO. The incidence of sleep disordered breathing symptoms in children from 2 to 19 years of age. J Am Ortho Soc. 2016;16(1):24-28.
2. Chervin RD, Hedger K, Dillon J, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21-32.
3. Peña-Zarza JA, Osona-Rodriguez de Torres B, Gil-Sanchez JA, Figuerola-Mulet J. Utility of the pediatric sleep questionnaire and pulse oximetry as screening tools in pediatric patients with suspected obstructive sleep apnea syndrome. Sleep Disord. 2012;2012:819035.
4. Brouilette R, Hanson D, David R, et al. A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr. 1984;105(1):10-14.
5. Villa MP, Bernkopf E, Pagani J, et al. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am J Respir Crit Care Med. 2002;165(1):123-127.
6. Guilleminault C, Sullivan SS. Towards restoration of continuous nasal breathing as the ultimate treatment goal in pediatric obstructive sleep apnea. Enliven: Pediatrics Neonatal Biol. 2014;1(1):1-5.
7. Lee SY, Guilleminault C, Chiu HY, Sullivan SS. Mouth breathing, "nasal disuse," and pediatric sleep-disordered breathing. Sleep Breath. 2015;19(4):1257-1264.
8. Torre C, Guilleminault C. Establishment of nasal breathing should be the ultimate goal to secure adequate craniofacial and airway development in children. J Pediatr (Rio J). 2018;94(2):101-103.
9. Rappai M, Collop N, Kemp S, deShazo R. The nose and sleep-disordered breathing: what we know and what we do not know. Chest. 2003;124(6):2309-2323.
10. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen. Allergy. 2008;63(suppl 86):8-160.
11. Mir E, Panjabi C, Shah A. Impact of allergic rhinitis in school going children. Asia Pac Allergy. 2012;2(2):93-100.
12. Massler M, Zwemer JD. Mouth breathing. II. Diagnosis and treatment. J Am Dent Assoc. 1953;46(6):658-671.
13. Bergersen EO. A review of recent research studies on sleep-disordered breathing in children relevant to the dental professionals. Compend Contin Educ Dent. 2020;41(6):e1-e13.
14. Josell SD. Habits affecting dental and maxillofacial growth and development. Dent Clin North Am. 1995;39(4):851-860.
15. Wada H, Kimura M, Tajima T, et al. Nocturnal enuresis and sleep disordered breathing in primary school children: potential implications. Pediatr Pulmonol. 2018;53(11):1541-1548.
16. Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153(1):255-259.
17. Finkelstein Y, Wexler D, Berger G, et al. Anatomical basis of sleep-related breathing abnormalities in children with nasal obstruction. Arch Otolaryngol Head Neck Surg. 2000;126(5):593-600.
18. Koren D, Gozal D, Bhattacharjee R, et al. Impact of adenotonsillectomy on insulin resistance and lipoprotein profile in nonobese and obese children. Chest. 2016;149(4):999-1010.
19. Gozal D, Kheirandish-Gozal L. The multiple challenges of obstructive sleep apnea in children: morbidity and treatment. Curr Opin Pediatr. 2008;20(6):654-658.
20. Villa MP, Castaldo R, Miano S, et al. Adenotonsillectomy and orthodontic therapy in pediatric obstructive sleep apnea. Sleep Breath. 2014;18(3):533-539.
21. Kheirandish-Gozal L, Dayyat EA, Eid NS, et al. Obstructive sleep apnea in poorly controlled asthmatic children: effect of adenotonsillectomy. Pediatr Pulmonol. 2011;46(9):913-918.
22. Mitchell RB, Kelly J. Behavioral changes in children with mild sleep-disordered breathing or obstructive sleep apnea after adenotonsillectomy. Laryngoscope. 2007;117(9):1685-1688.
23. Huang YS, Guilleminault C, Lee LA, et al. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. 2014;37(1) 71-76.
24. Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109(12):1901-1907.
25. Passalacqua G, Albano M, Canonica GW, et al. Inhaled and nasal corticosteroids: safety aspects. Allergy. 2000;55(1):16-33.
26. Villa MP, Malagola C, Pagani J, et at. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007;8(2):128-134.
27. Ieto V, Kayamori F, Montes MI, et al. Effects of oropharyngeal exercises on snoring: a randomized trial. Chest. 2015;148(3):683-691.
28. Villa MP, Brasili L, Ferretti A, et al. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath. 2015;19(1):281-289.
29. Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38(5):669-675.
30. Bergersen, EO. Sleep disordered breathing questionnaire for young children. J Am Ortho Soc. Fall 2015;14-18.
31. Urschitz MS, Eitner S, Guenther A, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics. 2004;114(4):1041-1048.
32. Sahin U, Ozturk O, Ozturk M, et al. Habitual snoring in primary school children: prevalence and association with sleep-related disorders and school performance. Med Princ Pract. 2009;18(6):458-465.
33. Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153(2):866-878.
34. Barr L, Thibeault SL, Muntz H, de Serres L. Quality of life in children with velopharyngeal insufficiency. Arch Otolaryngol Head Neck Surg. 2007;133(3):224-229.
35. Attanasio R, Bailey DR. Dental Management of Sleep Disorders. Ames, Iowa: Wiley-Blackwell; 2010.
36. Lee SH, Choi JH, Shin C, et al. How does open-mouth breathing influence upper airway anatomy? Laryngoscope. 2007;117(6):1102-1106.
37. Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest. 1995;107(4):963-966.
38. Neill AM, Angus SM, Sajkov D, McEvoy RD. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1997;155(1):199-204.
39. Oksenberg A, Silverberg DS, Arons E, Radwan H. The sleep supine position has a major effect on optimal nasal continuous positive airway pressure. Chest. 1999;116(4):1000-1006.
40. Xiheng G, Chen W, Hongyu Z, et al. The study of the influence of sleep position on sleep apnea. Sci J American Assoc for Resp Care. 2003;1-2.
41. Szollosi I, Roebuck T, Thompson B, et al. Lateral sleeping position reduces severity of central sleep apnea/Cheyne-Strokes respiration. Sleep. 2006;29(8):1045-1051.
42. Dayyat E, Maarafeya MA, Capdevila OS, et al. Nocturnal body position in sleeping children with and without obstructive sleep apnea. Pediatr Pulmonol. 2007;42(4):374-379.
43. Cuhadaroglu C, Keles N, Erdamar B, et al. Body position and obstructive sleep apnea syndrome. Pediatr Pulmonol. 2003;36(4):335-338.
44. Gozal D, Kheirandish-Gozal L. Obesity and excessive daytime sleepiness in prepubertal children with obstructive sleep apnea. Pediatrics. 2009;123(1):13-18.
45. Adamidis IP, Spyropoulos MN. The effects of lymphadenoid hypertrophy on the position of the tongue, the mandible and the hyoid bone. Eur J Orthod. 1983;5(4):287-294.
46. Behlfelt K. Enlarged tonsils and the effect of tonsillectomy. Characteristics of the dentition and facial skeleton. Posture of the head, hyoid bone and tongue. Mode of breathing. Swed Dent J Suppl. 1990;72:1-35.
47. Dayyat E, Kheirandish-Gozal L, Capdevila OS, et al. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136(1):137-144.
48. Sillman JH. Development of occlusion; a serial study from birth to seven years. J Second Dist Dent Soc. 1945;31:153-163.
49. Bergersen EO. The directions of facial growth from infancy to adulthood. Angle Orthod. 1966;36(1):18-43.
50. Kim YJ, Hong JS, Hwang YI, Park YH. Three-dimensional analysis of pharyngeal airway in preadolescent children with different anteroposterior skeletal patterns. Am J Orthod Dentofacial Orthop. 2010;137(3):306e1-e11.
51. Methenitou S, Shein B, Ramanathan G, Bergersen EO. Prevention of overbite and overjet development in the 3 to 8 year old by controlled nighttime guidance of incisal eruption: a study of 43 individuals. J Pedod. 1990;14(4):219-230.