Pediatric Phantom Dosimetry of a Portable Handheld X-Ray Device
Juan F. Yepes, DDS, MD, MPH, MS, DrPH; Jackie Hwang, DMD; LaQuia A. Vinson, DDS, MPH; Brian J. Sanders, DDS, MS; James E. Jones, DMD, MSD, EdD, PhD; K. Brandon Johnson, RDH, MS; and Qing Tang, MS
Abstract
Purpose:The purpose of this study was to quantify radiation dose from the XTG (Xray2Go) handheld x-ray device for bitewing and maxillary anterior occlusal projections using a pediatric phantom. The aim was to evaluate effects of thyroid shielding on total effective dose (E) and tissue equivalent doses (HT) and assess operator backscatter radiation. Methods:A pediatric phantom head with 24 tissue site dosimeters was exposed to radiation from the x-ray device. Exposures included: (1) right and left bitewing (BW) without thyroid collar on phantom, (2) BW with thyroid collar, (3) maxillary anterior occlusal (AO) without thyroid collar, (4) AO with thyroid collar. With each exposure type, new dosimeter sets were used and 30 exposures completed. The operator wore dosimeters on the forehead and right hand to quantify backscatter radiation. Average values of HT and E were calculated. Results:Thyroid shielding produced a statistically significant difference for posterior BW projections at thyroid (P = .0002), lymphatic nodes (P = .0367), and muscle tissue sites (P = .0367). Backscatter radiation from the x-ray device was indistinguishable from background radiation. Conclusions:Thyroid shielding made a statistically significant difference for radiation dose with the Xray2Go for BW projections at specific tissue sites, including the thyroid, lymph nodes, and muscle, and for overall effective dose. Radiation to the operator from the device was very low and indistinguishable from background radiation.
Radiographs are an essential component of dental practice, allowing dentists to diagnose dental caries, identify orofacial pathology, and evaluate dental development. Traditional dental radiograph systems are fixed, wall-mounted systems; however, handheld alternatives have been developed in the past two decades.1 Handheld digital radiography represents a paradigm shift in the way dental radiographic images are obtained,2 offering portability and cost advantages while still producing diagnostic-quality radiographic images.3
With this novel technology, initial concerns arose regarding unintended exposure of the operator to backscatter radiation from the handheld device due to proximity during operation4 and potential increased radiation exposure to patients.5 These potential issues prompted numerous evaluations of one prominent handheld radiology device, the NOMAD™ (Aribex Inc). Several radiation backscatter studies as well as phantom dosimetry studies have been completed, validating the NOMAD's safety for both patient and operator.2,4,6-9
Assessments of ionizing radiation can be accomplished by radiation dosimetry, in which a dosimeter registers the amount of radiation absorbed at a given target. To elucidate the associated health risk of the absorbed dose detected by dosimeters, studies often calculate the overall effective dose. The effective dose is the preferred measurement, as stated by the International Commission on Radiological Protection (ICRP), to compare risk from different radiographic examinations. This value considers the variable radiation sensitivity of different tissues in human bodies and modifies the absorbed dose with a tissue weighting factor.10 The effective dose (E) is a conceptual measurement of the whole-body risk of future health detriment, or possible cancer induction, from ionizing radiation exposure. An E of 1 sievert (Sv) represents approximately a 5.5% chance of developing cancer.10
Dosimeters that register absorbed dose can be worn by an operator or housed within an imaging phantom, a dummy apparatus designed to simulate the patient. A pediatric imaging phantom is composed of materials that approximate the density and responsiveness of tissues of an average 10-year-old pediatric human head and has slots designed to hold dosimeters in strategic positions within the anthropomorphic model.11,12 When the phantom head is exposed to ionizing radiation, the encased dosimeters detect the absorbed dose of radiation at these orofacial sites of interest. Studies utilizing a pediatric phantom are important, as pediatric patients have greater risk associated with ionizing radiation due to the sensitivity of developing organs to radiation and a longer remaining lifetime over which a radiation-induced cancer could present.13
Completion of phantom studies, particularly pediatric phantom studies, with handheld devices is critical for assessing patient safety and understanding how their doses compare to existing research on traditional imaging equipment. Many handheld radiology units are currently available on the market. Some are not FDA approved and may produce potentially hazardous amounts of radiation.14 Thus, it is imperative that providers verify the safety of the equipment they are using.
The XTG (Xray2Go) Handheld X-ray (Digital Doc, LLC, digi-doc.com) is a new lightweight portable device that can be operated like a camera. While the device has FDA approval, there are no published independent evaluations of radiation exposure for pediatric patients or operators.
The primary aim of this study was to quantify the effective dose (E) and tissue equivalent dose (HT) in microsieverts (μSv) at tissue sites of interest within a pediatric anthropomorphic phantom head, with and without a protective thyroid collar, when exposed to left and right bitewing (BW) and maxillary anterior occlusal (AO) radiographs using the XTG (Xray2Go) Handheld X-ray device. In addition, the authors evaluated the amount of backscatter radiation for an operator while using the device.
Materials and Methods
This dosimetry study was completed using a pediatric phantom modeling the anatomy of a 10-year-old child (ATOM® model 706 HN, CIRS Inc, cirsinc.com) (Figure 1). Modifications were made to the phantom, with pockets to hold dosimeters at 24 sites of interest (Figure 2, Table 1). The dosimeter pockets positioned in the head and neck corresponded to tissues of interest in the 2007 Recommendations of the ICRP. Placement of the neck dosimeters were vertically centered to the slice and taped in place. Dosimeter placement for the lens of the eye corresponded to the anatomical lens location and was also taped in position. Dosimeters housed within the phantom apparatus were standardized in position by maintaining the uppermost edge of the dosimeter at the level of the superior plane of the designated slice and retained in position by resistance of the dosimeter case within its designated slot.12 In addition to the dosimeters placed within the phantom head, two dosimeters were worn by the operator to record backscatter radiation. These dosimeters were placed on the center of the forehead and dorsal side of the right hand of the operator and taped into position.
The dosimeters used for this study were 1 mm x 10 mm x 10 mm optically stimulated luminescent dosimeters (OSLDs) (nanoDot™, Landauer, landauer.com), which were enclosed in opaque light-tight plastic holders to prevent ambient light exposure during transport. The authors used a calibrated portable dosimeter reader (microSTAR®ii, Landauer) to process the dosimeters after exposure, at the University of North Carolina, Chapel Hill.
The pediatric phantom head was subjected to radiographic exposures that are typical in pediatric practice: bitewings and maxillary anterior occlusal radiographs. The experiment was run with the Xray2Go unit's fixed settings of 60 kVp tube voltage and 2mA tube current, and an adjustable exposure time that was set at 0.06 seconds as recommended by the manufacturer for both of the chosen projections. The settings for the device are appropriate and fall within the optimal range of 60 to 70 kVp for dental radiographs, as stated by the American Dental Association.15 Positioning of the Xray2Go was controlled with the use of a customized positioning device, to allow for more consistent angulation of the handheld x-ray device during operation. A control set of dosimeters was utilized to record the background radiation from phantom transport. Background radiation refers to the persistent low level of radiation found in the environment from both man-made and natural sources, such as minerals in the soil, water, and cosmic radiation. These control dosimeters functioned to give the authors a baseline of background radiation and were excluded from radiation exposure from the experiment.
A total of 14 dosimeter sets were used for the following: Sets 1, 2, 3: 30 exposures on the patient's right for a right BW radiograph, and 30 exposures on the patient's left for a left BW radiograph with the patient phantom wearing a thyroid collar. Sets 4, 5, 6: 30 right BW exposures, and 30 left BW exposures without the phantom wearing a thyroid collar. Sets 7, 8, 9: 30 anterior maxillary occlusal radiograph exposures, with the phantom wearing a thyroid collar. Sets 10, 11, 12: 30 anterior maxillary occlusal radiograph exposures, without the phantom wearing a thyroid collar. Sets 13, 14: A pair of dosimeters were used to record backscatter radiation potentially affecting the operator, placed on the operator's forehead and right hand.
The operator stood at a designated point demarcated on the floor for each exposure. Floor markings indicated the foot position of the operator where, when standing at this position, the operator could comfortably hold the device's x-ray emitting cone flush with the positioning device. The operator's arms were slightly bent, and the backscatter shield of the x-ray unit was parallel to the operator as recommended by the Xray2Go user manual. The operator wore a protective lead apron with a thyroid collar for all exposures.
After completion of all exposure sets, dosimeters were processed by a commercial dosimeter reader (microSTARii) at the University of North Carolina, Chapel Hill. Values obtained from each dosimeter were divided by 30 to indicate the average absorbed dose per exposure, in micrograys. Absorbed dose was translated to equivalent dose (HT) by multiplying absorbed dose by radiation weighting factor, value = 1, for x-rays. This incurred no numerical change but signified a unit change from micrograys (µGy) to microsieverts (µSv), reflecting the type of ionizing radiation used. Effective dose (E) was determined by multiplying equivalent doses by their appropriate tissue weighting factors as determined by ICRP 2007 and determining the whole-body sum of these values. Doses were compared between data sets with and without thyroid collar.
Dosimeter readings of Xray2Go from 16 locations of the phantom, derived from the 24 dosimeter sites (as specified in Table 2), were analyzed using one-way ANOVA, with the factor for thyroid collar to identify its effect. All pair-wise group comparisons were made using Fisher's Protected Least Significant Differences used to control the overall significance level of pair-wise comparisons at 5%. Analyses were performed using SAS version 9.4 (SAS Institute, sas.com).
Results
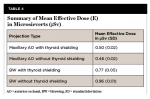
Dosimeter readings from 16 tissue categories, derived from 24 dosimeters on the phantom (Table 2) and two locations on the operator, were analyzed and recorded. Table 3 summarizes the mean, standard deviation (SD), standard error (SE), 95% confidence interval for the mean, and range of tissue equivalent doses in microsieverts (µSv) delivered by the Xray2Go unit for the various projection types, with and without thyroid shielding. The overall average effective dose of the BW projections was 0.77 μSv (SD = 0.05) with thyroid shielding and 0.96 μSv (SD = 0.01) without thyroid shielding. The average effective dose of the anterior occlusal projection was 0.50 μSv (SD = 0.02) with thyroid shielding and 0.46 μSv (SD = 0.02) without thyroid shielding (Table 4). Operator radiation registered from dosimeters on the forehead and hand was determined to be very low and indistinguishable from the dosimeter readings for background radiation. The value obtained from operator hand and forehead dosimeters did not differ significantly from the control dosimeters that were unexposed; these unexposed control dosimeters were set aside to detect any low levels of radiation from natural environmental exposure.
The highest average tissue equivalent dose from the BW without the phantom wearing the thyroid collar was for the salivary glands (mean [M] = 20.66 μSv, SD = 0.44), followed by the oral mucosa (M = 19.14 μSv, SD = 0.19), extrathoracic airway (M = 13.23 μSv, SD = 0.27), and thyroid (M = 8.48 μSv, SD = 0.31). The highest average tissue equivalent dose for the BW with the phantom wearing thyroid collar was for the salivary glands (M = 20.30 μSv, SD = 1.55), followed by oral mucosa (M = 18.87 μSv, SD = 1.38), extrathoracic airway (M = 12.14 μSv, SD = 0.88), and thyroid (M = 4.67 μSv, SD = 0.36). For the maxillary AO projection without the thyroid collar, highest average tissue equivalent dose was to lens of the eyes (M = 54.76 μSv, SD = 15.55), followed by the salivary glands (M = 11.20 μSv, SD = 0.47), extrathoracic airway (M = 9.04 μSv, SD = 0.62), and oral mucosa (M = 8.17 μSv, SD = 0.28). For the maxillary AO projection with the thyroid collar, highest average equivalent dose was to the lens of the eyes (M = 40.56 μSv, SD = 3.28), followed by the salivary glands (M = 12.28 μSv, SD = 0.47), extrathoracic airway (M = 9.11 μSv, SD = 0.11), and oral mucosa (M =8.94 μSv, SD = 0.28).
Figure 3 summarizes the exposure comparisons with and without thyroid shielding. Thyroid shielding made a statistically significant difference in reducing radiation dose for select locations in the phantom during the posterior BW projection. These locations included the thyroid (P =.0002), lymphatic nodes (P =.0367), and muscle (P =.0367). This was also the case for the overall effective dose (E) (P =.0033). For the maxillary AO projection, thyroid shielding did not produce a statistically significant difference for most tissue sites, except for the locations of the salivary glands (P =.0479) and oral mucosa (P =.0282). Interestingly, the dose in μSv with the phantom wearing a thyroid collar for the maxillary AO projection was higher than without thyroid shielding at these two locations.
Discussion
This was the first study to evaluate the tissue equivalent dose and overall effective dose produced by the XTG device for an anthropomorphic pediatric phantom, with and without a thyroid collar present on the phantom. The principles of ALARA (as low as reasonably achievable) offer guidance to clinicians in making thoughtful decisions about radiation exposure, bearing in mind the stochastic effects of radiation with increased probability of a radiation-induced health effect over time. In the pediatric population, careful imaging techniques and practices are even more important due to the increased radio-sensitivity of children.13 A pediatric phantom study by Yepes et al with the Kodak 9000 CBCT found that children receive one to three times more radiation and up to 10 times more radiation than adults for mandibular and maxillary CBCT scans, respectively.11 Typical dental radiation dose is quite low relative to other medical scans; however, with a lifetime frequency of dental imaging with some regularity, minimizing the dose can mitigate some risk. With potential for greater additive effects over a longer lifetime, pediatric imaging decisions are especially important.
The adoption of portable radiology is an opportunity to image more prudently in terms of patient exposure, as compared to reported effective doses from similar projections with wall-mounted imaging devices. Pharaoh and White cited that the average effective dose for traditional wall-mounted imaging with bitewings with rectangular collimation and F-speed film is 5 μSv.16 A 2016 adult phantom dosimetry study by Granlund et al that evaluated both panoramic imaging and intraoral imaging found the effective dose of a four bitewing projection from a wall-mounted device, the Gendex™ Oralix DC®, to be 3.4 μSv. The highest organ doses were found to be for the salivary glands and the mucosa, as was seen in the present study.17 An earlier study in 2014 used a pediatric phantom to assess the wall-mounted Gendex unit and found that effective dose for bitewings ranged from 1.5 to 2.7 μSv for a 10-year old anthropomorphic model.18 These values contrast with from those from the present study, which shows that with the portable XTG unit, effective dose for posterior bitewings was 0.77 μSv (SD = 0.05) with thyroid shielding and 0.96 μSv (SD = 0.01) without thyroid shielding. The values from the present study of the XTG unit for bitewing projections, even without thyroid shielding, were far less than estimates from traditional imaging devices.
It is important to note that the present authors cannot make direct comparisons, as there are factors that contribute to these differences, including technique factor settings, projection strategy, and phantom type. The Gendex studies had settings of 60 kVp and 7 mA or 65 kVp and 7 mA, respectively, for exposures, different exposure times, and performed exams on different imaging phantoms.17,18 The XTG device in the present study had fixed settings of 60 kVp and 2 mA and was consistently operated at 0.06 seconds, a time setting that fell within the manufacturer recommended range for exposures for digital imaging for these projections with this device. Acknowledging the inherent differences between these studies, the values reported for pediatric imaging from the XTG device are lower than those from traditional imaging devices and support patient safety of the device.
Thyroid shielding is a simple way to reduce dose to the patient, as shown in the bitewing assessment in the present study. Thyroid shielding significantly reduced the overall effective dose for the bitewing projections, and was also strongly reflected in specific tissue site equivalent doses as well, including the thyroid, lymphatic nodes, and muscle. With the thyroid noted as one of the most radiosensitive organs in the head and neck area,10 implementation of this precaution to limit exposure to this area is a simple modification with significant benefits. When it comes to the present results regarding the anterior occlusal projection, several variables could account for the unexpected findings of higher doses with thyroid shielding rather than without. Non-ideal placement of the thyroid collar against the phantom model could have impacted radiation dose. In addition, although efforts were made to standardize operator position for the anterior occlusal projection during the XTG study, inevitable operator positioning shifts could have occurred between exposures. A 2018 study by Worrall et al that examined the effect of thyroid collar on dose reduction for an anterior occlusal view found that suboptimal examination position can increase thyroid dose significantly, even with a phantom wearing a collar; in those scenarios, the thyroid was in the path primary beam, while shielded, due to angulation.19 The factor of suboptimal operator positioning and angulation could account for some of the difference observed in the present study. Future studies with a custom thyroid collar that fits the phantom ideally, and fixing the device on an immobile tripod rather than using an active operator, could optimize the study, although such changes would not reflect the true clinical application of the device. Clinicians should be aware of the benefits of thyroid shielding but mindful of their position when imaging, so as not to inadvertently include the thyroid in the primary beam.
There are currently very few dosimetry studies of handheld radiology devices that assess patient dose using anthropomorphic phantom dosimetry, however multiple studies exist that discuss operator safety. Further investigations need to be completed with other portable dental radiology devices as well as repeat studies to validate previous findings. Dentists would benefit from a uniform investigation of multiple devices to understand how the estimated patient effective dose of the XTG compares to others.
Studies on backscatter dose have supported the safety of handheld devices for the operator. Studies of the NOMAD and other portable dental x-ray devices have shown that portable units satisfy the principles of ALARA for operator exposure, with doses well below 1 mSv per year, or 2% of the annual occupational dose limit.7 A 2012 study by Gray et al corroborates this idea, stating that doses to dental staff for handheld devices are far lower than those from a wall-mounted system.8 This contrasts with a 2019 study by Smith et al, which stated concerns with stray radiation to the operator and recommended limiting handheld device usage to cases where accessibility demands portable device use.20 The present study reported that dose to the operator from the XTG was so low that it was indistinguishable from background radiation. The XTG unit has a built-in safety feature that includes a collimator cone and 6-inch diameter backscatter shield that likely contribute greatly to the low operator dose.
While the present study was able to quantify the absorbed, equivalent, and effective dose from the XTG unit for a pediatric phantom, it did not assess the diagnostic quality of images produced by the device. An image quality study by Pittayapat et al found that portable dental x-ray units show good diagnostic imaging for a variety of devices, including MinXRay® (MinXray, Inc, minxray.com), AnyRay (Vatech, vatech.es), Rextar (RF America IDS, myrfamerica.com), and NOMAD devices.3 Similarly, a 2020 comparative study by Nitschke et al showed that the NOMAD™ Pro 2 (Kavo, kavo.com) device delivered comparable image quality as a wall-mounted device.21 These studies did not include the XTG device. More studies on image quality of portable devices, including the XTG, should also be completed to understand this aspect of their comparison to wall-mounted devices. It appears, however, that with regard to reducing operator and patient radiation risk, the portable device used in this study is a sensible option for imaging.
Conclusion
Based on this study, the authors conclude that: (1) operator backscatter radiation dose to the forehead and hand from the handheld x-ray device tested (Xray2Go) was minimal and indistinguishable from background radiation, and (2) thyroid shielding made a statistically significant difference in reducing radiation dose from bitewing projections for the thyroid, lymph nodes, muscle, and overall effective dose when using this device.
Acknowledgment
The research leading to these results was possible with use of the pediatric phantom, optically stimulated luminescent dosimeters, and dosimeter reader loaned from the North Carolina Oral Health Institute in Chapel Hill, North Carolina.
Disclosure
The authors had no disclosures to report.
About the Authors
Juan F. Yepes, DDS, MD, MPH, MS, DrPH
Professor, Department of Pediatric Dentistry, Indiana University School of Dentistry, Indianapolis, Indiana
Jackie Hwang, DMD
Pediatric Dentist, Indiana University/Riley Hospital for Children, Indianapolis, Indiana
LaQuia A. Vinson, DDS, MPH
Associate Professor and Program Director, Department of Pediatric Dentistry, Indiana University School of Dentistry, Indianapolis, Indiana
Brian J. Sanders, DDS, MSProfessor and Chairperson, Department of Pediatric Dentistry, Indiana University School of Dentistry, Indianapolis, Indiana
James E. Jones, DMD, MSD, EdD, PhD
Paul E. Starkey Research Professor, Department of Pediatric Dentistry, Indiana University School of Dentistry, Indianapolis, Indiana
K. Brandon Johnson, RDH, MS
Assistant Professor, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
Qing Tang, MS
Biostatistician, Department of Biostatistics, Indiana University School of Medicine, Indianapolis, Indiana
References
1. Hosseini Pooya SM, Hafezi L, Manafi F, Talaeipour AR. Assessment of the radiological safety of a Genoray portable dental x-ray unit. Dentomaxillofac Radiol. 2015;44(3):20140255.
2. Aribex launches NOMAD Pro 2 in US. J Engineering. Apr 24, 2013;1185.
3. Pittayapat P, Oliveira-Santos C, Thevissen P, et al. Image quality assessment and medical physics evaluation of different portable dental X-ray units. Forensic Sci Int. 2010;201(1-3):112-117.
4. Danforth RA, Herschaft EE, Leonowich JA. Operator exposure to scatter radiation from a portable handheld dental radiation emitting device (Aribex NOMAD) while making 915 intraoral dental radiographs. J Forensic Sci. 2009;54:415-421.
5. Berkhout WER, Suomalainen A, Brullmann D, et al. Justification and good practice in using handheld portable dental x-ray equipment: a position paper prepared by the European Academy of DentoMaxilloFacial Radiology (EADMFR). Dentomaxillofac Radiol. 2015;44(6):20140343.
6. Goren AD, Bonvento M, Biernacki J, Colosi DC. Radiation exposure with the NOMAD portable x-ray system. Dentomaxillofac Radiol. 2008;37(2):109-112.
7. McGiff TJ, Danforth RA, Herschaft EE. Maintaining radiation exposure as low as reasonably achievable (ALARA) for dental personnel operating portable hand-held x-ray equipment. Health Phys. 2012;103(2 suppl 2):179-185.
8. Gray JE, Bailey ED, Ludlow JB. Dental staff doses with handheld dental intraoral x-ray units. Health Phys. 2012;102(2):137-142.
9. Rottke D, Gohlke L, Schrodel R, et al. Operator safety during the acquisition of intraoral images with a handheld and portable x-ray device. Dentomaxillofac Radiol. 2018;47(3):20160410.
10. Ludlow JB, Davies-Ludlow LE, White SC. Patient risk related to common dental radiographic examinations: the impact of 2007 International Commission on Radiological Protection recommendations regarding dose calculation. J Am Dent Assoc. 2008;139(9):1237-1243.
11. Yepes JF, Booe MR, Sanders BJ, et al. Pediatric phantom dosimetry of Kodak 9000 cone-beam computer tomography. Pediatr Dent. 2017;39(3):229-232.
12. Ludlow JB, Walker C. Assessment of phantom dosimetry and image quality of i-CAT FLX cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2013;144(6):802-817.
13. American Dental Association. Image Gently® campaign expands to dentistry: urges dental professionals to use child-size radiation dose. ADA website. September 26, 2014. https://www.ada.org/en/press-room/news-releases/2014-archive/september/images-gently-urges-dental-professionals-to-use-child-size-radiation-dose. Accessed March 15, 2021.
14. Mahdian M, Pakchoian AJ, Dagdeviren D, et al. Using hand-held dental x-ray devices: ensuring safety for patients and operators. J Am Dent Assoc. 2014;145(11):1130-1132.
15. American Dental Association. Dental Radiographic Examinations: Recommendations for Patient Selection and Limiting Radiation Exposure. ADA Council on Scientific Affairs. 2012:16.
16. White SC, Pharoah MJ, eds. Radiation safety and protection. In: Oral Radiology: Principles and Interpretation. 6th ed. St. Louis, MO: Mosby/Elsevier; 2009;35.
17. Granlund C, Thilander-Klang A, Ylhan B, et al. Absorbed organ and effective doses from digital intra-oral and panoramic radiography applying the ICRP 103 recommendations for effective dose estimations. Br J Radiol. 2016;89(1066):20151052.
18. Branets I, Stabulas J, Dauer LT, et al. Pediatric bitewing exposure to organs of the head and neck through the use of juvenile anthropomorphic phantoms. J Oral Bio. 2014;1(1):5.
19. Worrall M, Menhinick A, Thomson DJ. The use of a thyroid shield for intraoral anterior oblique occlusal views-a risk-based approach. Dentomaxillofac Radiol. 2018;47(1):20170140.
20. Smith R, Tremblay R, Wardlaw GM. Evaluation of stray radiation to the operator for five hand-held dental x-ray devices. Dentomaxillofac Radiol. 2019;48(5):20180301.
21. Nitschke J, Schorn L, Holtmann H, et al. Image quality of a portable x-ray device (Nomad Pro 2) compared to a wall-mounted device in intraoral radiography. Oral Radiol. 2020. doi: 10.1007/s11282-020-00434-1.