Assessment of Oral Human Papillomavirus Prevalence in a Multi-ethnic Pediatric Clinic Population
Vikram Tiku, DDS; Chelsie J. Todd; and Karl Kingsley, PhD, MPH
Abstract
The human papillomavirus (HPV) family has been associated with many cancers, including oral cancer. Recent studies have also revealed HPV presence from healthy adult and pediatric patient saliva samples, though much less is known about the oral prevalence of high-risk HPV in healthy children and adolescents. The primary goal of this project was to assess the prevalence of HPV within a pediatric dental clinic using noninvasively collected saliva. In brief, saliva was collected and DNA isolated to screen for HPV strains HPV 6, HPV 11, HPV 16, and HPV 18. Screening of these samples (N = 187) revealed 9.2% (n = 19/187) harbored one or more HPV strains. Although the proportion of saliva samples from males:females and minorities:non-minorities harboring HPV was not significantly different from the overall sample, an analysis of age revealed significantly more samples harboring HPV were found among the youngest age cohorts (3 to 5 years > 6 to 11 years > 12 to 17 years). In addition, most samples harbored HPV16 (n = 13/19 or 68.4%). These findings suggest oral HPV infection may, in fact, be more prevalent than previously described, which intimates that knowledge and awareness of the potential health effects and benefits of HPV vaccination may be appropriate for these patients.
The family of DNA human papillomaviruses (HPV) have been implicated in many types of cancer.1 The most widely known HPV-associated cancer is gynecological or cervical cancer; however, recent evidence has also implicated HPV in the etiology and pathogenesis of other cancers, including in the oral cavity.2-5 In fact, two recent studies demonstrated the proportion of oral tumors harboring HPV has increased in the past three decades and is becoming a cause for concern among dentists and other oral health professionals.6,7
While the relationships and associations between HPV infection and oral-cancer phenotypes have been the subject of investigation,8,9 retrospective analyses of oral saliva samples collected from healthy adult and pediatric populations have also revealed high-risk HPV infection.10,11 Although these studies found that gender and race were associated with oral HPV presence, the retrospective nature of the studies did not allow for more detailed analysis of associations with patient demographics, health status, or other variables of interest.
Many studies of oral HPV have been conducted; however, they have more narrowly focused on two main areas. The first is whether an association exists between children infected with the human immunodeficiency virus (HIV) and the presence of oral HPV.12,13 The second area of study involves whether the oral HPV status of vaginally delivered newborns is associated with maternal HPV infection.14,15 Much less information is known about the oral prevalence of high-risk HPV in young children and adolescents, excluding neonates and HIV-infected children. The few studies in this area have reported widely differing results and outcomes.16,17
Purpose/Objective
Based on a paucity of evidence, as well as contradictory outcomes described in previously published studies, the primary objectives of this study were to assess the prevalence of high-risk oral HPV within a pediatric dental population and provide associated detailed demographic and clinical patient data from this sample.
Materials and Methods
Human Subjects
A cross-sectional observational prospective research design was used to conduct this study. A convenience sample of pediatric patients were recruited from the University of Nevada Las Vegas (UNLV) School of Dental Medicine clinics. Pediatric dental residents recruited UNLV pediatric subjects between the ages of 3 and 17 years after receiving informed consent from parents or guardians for their children to participate in the study. Although children under 18 years of age are not able to give informed consent, in Nevada, children aged 7 years and older who are able to read, comprehend, and write are asked to provide “pediatric assent,” which is an agreement to voluntarily participate in research. Pediatric assent from each patient was also obtained prior to collection of demographic data and saliva samples. Patients whose parents or guardians declined to let them participate were excluded, as were patients who themselves declined to participate. Also, any child who was not a patient of record at the UNLV School of Dental Medicine clinics was excluded. The protocol for this study, “The Prevalence of Oral Microbes in Saliva from the UNLV School of Dental Medicine pediatric and adult clinical population,” (Protocol #:1305-4466M) was reviewed and approved by the University of Nevada Las Vegas (UNLV) Office of Research Integrity – Human Subjects (ORI – HS) on June 4, 2013.
Saliva Collection
Saliva samples were collected from participants in small, sterile saliva 50-mL collection containers (Fisher Scientific, fishersci.com). In brief, patients were asked to expectorate to provide at least 0.5 mL, which resulted in sample volumes that varied from approximately 0.5 mL to 5.0 mL, as previously described.18 Samples were stored on ice until transfer to a biomedical laboratory for analysis within 2 hours of collection. Each patient sample was given a unique, randomly generated number as an identifier to prevent research bias. The corresponding demographic and health information was simultaneously collected and given the same randomly generated number to facilitate analysis, but no identifying patient information was available to the research team.
Screening and Analysis
DNA was isolated from each sample using the GenomicPrep DNA isolation kit (Amersham Biosciences, gelifesciences.com) according to the procedure recommended by the manufacturer for fluid and tissue analysis.18 DNA was re-suspended and stored in 50-uL DNA hydration solution at 4°C. DNA purity was calculated using ratio measurements of absorbance at 260 nm and 280 nm (A260/A280 ratio), which ranged between 1.4 and 2.0. DNA from each sample was then used to perform polymerase chain reaction (PCR) screening using the Fisher exactGene™ Complete PCR kit (Fisher Scientific) and a Mastercycler® gradient thermocycler (Eppendorf, eppendorf.com) using the primers listed in Table 1 for HPV 6, HPV 11, HPV 16, and HPV 18, which were synthesized by SeqWright (gelifesciences.com).10,11
One microgram of template DNA was used for each reaction. The initial denaturation step ran for 3 minutes at 94°C. A total of 30 amplification cycles were run, consisting of 30 seconds of denaturation at 94°C, 60 seconds of annealing (HPV 6 = 61.6°C; HPV 11 = 63.7°C; HPV 16 = 58.4°C; HPV 18 = 54.9°C), and 30 seconds of extension at 72°C. Final extension was run for 5 minutes at 72°C. The PCR reaction products were separated by gel electrophoresis using Reliant™ 4% NuSieve® 3:1 Plus Agarose gels (Lonza, lonza.com). Bands were visualized by ultraviolet illumination of ethidium-bromide–stained gels and captured using a Kodak Gel Logic 100 Imaging System (Eastman Kodak, kodak.com) and 1D Image Analysis Software (Eastman Kodak).
Sensitivity and Specificity
DNA samples were then processed using qPCR to provide analysis and quantification of specificity and sensitivity. Standard curves for HPV16 detection were developed using DNA extracted from CaSki (ATCC® CRL-1550™: 600 copies/genome) (American Type Culture Collection [ATCC], atcc.org) and for HPV18 detection using GH354 (ATCC CRL-13003™: 200 copies/genome) (ATCC). DNA extracted from CaSki and GH354 cells were serially diluted tenfold starting at 50 ng to 0.0005 ng. Quantification was achieved using Cycle Threshold (CT) measured with the second derivative maximum method (LightCycler® 480 Software version 1.5.0.39, Roche Applied Sciences, lifescience.roche.com).
In brief, primers and probes were designed using Roche Universal Probelibrary (UPL) assay design software to amplify the region overlapping E6 and E7 gene sequence of HPV 16 and HPV 18. These primers were purchased from Sigma-Aldrich (sigmaaldrich.com) and probes from Roche Applied Science. HPV 16 E6/E7 forward primer 50-CAACTGATCTC TACTGTTATGAGCAA-30, HPV 16 E6/E7 reverse primer 50-CCAGCTGGACCATCTATTTCA-30, HPV 16 E6/E7 hydrolysis “Taqman” probe 50-(fam)-AGGAGGAG-(dark quencher dye)-30 (UPL probe No. 63) was used to amplify the 73 base pair (bp) region between the 535-nucelotide (nt) and 607-nt positions. HPV 18 E7 forward primer 50-GACTCAGAGGAAGGAAAACGATGAAA, HPV18 E7 reverse primer 50-GTGACGTTGTGGTTCGGCT; HPV 18 E7 probe 50-TGGAGTTAATCATCAACATT TACCA was used to amplify the 25-bp region between the 715-nt and 739-nt positions. The real-time reaction mixture was prepared in a LightCyclerW 480 multiwell Plate 96 containing 1x LightCyclerW 480 Probes Master (Roche Applied Sciences), 1 μm of each respective primer set (forward and reverse), 0.2 μm of respective probe, and 2 μl of DNA template; in a 20-μl final reaction volume. The probes master mix contained reaction buffer, dNTP mix (including dUTP in place of dTTP), 3.2 mM MgCl2, and Taq DNA polymerase. The realtime PCR assay was performed on a LightCycler 480 system (Roche Applied Sciences) with the following cycle parameters: preincubation for initial enzyme activation at 95°C for 10 minutes, followed by 45 cycles of 95°C for 10 seconds (ramp rate 4.4°C/second), 60°C for 30 seconds (ramp rate 2.2°C/second) and 72°C for 1 second (ramp rate 4.4°C/second). Following amplification, a cooling step was performed at 40°C for 30 seconds (ramp rate of 2.2°C per second). Acquisition of the fluorescence signal was performed using Mono Hydrolysis Probe setting (465 nm to 510 nm) following the 72°C extension phase of each cycle. All samples were carried out in triplicate.
Sensitivity and specificity were calculated as the proportion of true positives (>0.01 copies/genome) and true negatives (cutoff value > 0.001 copies/genome), respectively. Screening of HPV 16-positive samples against HPV 18 and HPV 18-positive samples against HPV 16 revealed specificity using this method for detection was 100%.10,11
Statistical Analysis
Sample size was calculated using the published recovery rate from the limiting step of DNA extraction (90% to 95%), which establishes the expected difference of 0.10 or 10%.19 The minimum sample size was then calculated using a significance level of α = 0.05 and P = .80, yielding a result of N = 50.20 Following the acquisition of saliva samples, DNA extraction, and HPV screening, demographic information from the subjects who provided these samples was compared with the overall demographic profile of the sample population and patient pool using chi-square (X2) to determine if any characteristics (race, gender, age) were different than expected, which may reveal selection bias.
Results
Saliva samples were collected from 206 clinic patients, who were nearly equally distributed between males and females, similar to the gender distribution from the overall UNLV pediatric clinic population (Table 2).21 Although most participants in this study and the clinic represented minority populations, the study sample contained slightly more (65.5%) than the overall clinic population (59.2%) and was, therefore, greater than would have been expected in a random selection (P < .01). More specifically, the percentage of Hispanics in the study sample (57.4%) was lower than in the overall clinic population (68.5%). Conversely, the percentage of Black participants in the study sample was higher (29.6%) than the percentage in the overall clinic population (13.1%).
The saliva samples were then processed to isolate DNA and screen for the presence of high-risk HPV strains (Table 3). DNA was successfully recovered from 187 of 206 samples, yielding a recovery rate of 90.8%, which is within (although at the low end) of the range specified by the manufacturer. Nineteen patient samples tested positive, representing 9.2% of the total sample (n = 19/187). More specifically, slightly more than half of positive samples were obtained from male subjects (n = 11/19 or 57.9%) (P < .01). Although no differences were found among the percentages of minority or non-minority subjects testing positive (P = .53), there were significantly more positive samples from the youngest age cohort (3 to 5 years, 31.5%) and fewer from the eldest cohort (12 to 17 years, 26.3%), P < .01.
Gender, age, and race/ethnicity (to some extent) associated with the presence of oral HPV within this patient sample. Age was negatively associated with the presence of oral HPV (R = -0.13) but was not statistically significant. Correlations with both gender and race were similarly non-significant (R = 0.093, 0.049, respectively). Finally, no association was found between body mass index (BMI) or decayed, missing, filled teeth (DMFT) score (R = -0.0213, 0.0073, respectively).
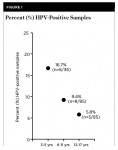
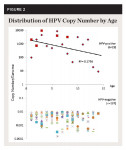
To facilitate comparison with other studies of oral HPV among children, these data were sorted and analyzed using corresponding age categories of 3 to 5, 6 to 11, and 12 to 17 years old (Figure 1). These analyses revealed that although 3- to 5-year-olds represented nearly one-third (31.5%) of HPV-positive samples, this proportion also represented nearly one-sixth of all 3- to 5-year-olds from the study sample (n = 6/36 or 16.7%). In addition, almost 10% of the 6- to 11-year-olds’ samples were HPV positive (n = 8/85), but only 5.8% of the 12- to 17-year-olds’ samples were HPV positive (n = 5/85). An assessment of the relative burden of HPV-infected cells, plotting the copy number per genome from each sample, is shown in Figure 2.
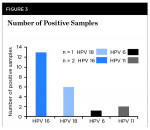
Finally, each of the strains of HPV detected from this analysis were graphed to demonstrate that HPV 16 (n = 13/19 or 68.4%) and HPV 18 (n = 6/19 or 31.6%) were detected (Figure 3). However, three of the samples also harbored additional HPV types. Specifically, one of the HPV 18-positive samples also contained HPV 6, and two of the HPV 16-positive samples also contained HPV 11 (Figure 3).
Discussion
The objective of this study was to assess the presence of high-risk HPV using saliva from healthy pediatric clinic patients and to explore associations with demographic and clinical data. The highest percentage of subjects with oral HPV was found in 3- to 5-year-olds, with decreasing prevalence among older subjects. These data confirm the pattern of age-inverse associations in oral HPV prevalence observed in previous screening studies, although the prevalence rates were markedly different.13,16 For example, evidence suggested the presence of oral HPV ranging from 7.9% to 16.2% in the sample of young children (aged 3 to 11) in this study, while other studies found HPV in nearly half of their subjects that were sampled (45.2%, 51.7%).22,23 However, all of these studies found that as children age, the detection of oral HPV decreased significantly, ranging from 5.1% to 16.2% in older children, ranging from 12- to 17-year-olds.13,16,22,23
Although the current study provides new evidence that oral HPV may be much higher (9.2%) than previous screenings from this research group have reported (2.5%), findings in this study suggest that the larger samples and the prospective nature of the current study may provide rationale for the differences in the observations reported here as compared to the previous report.11
It was hoped that an analysis of several variables of interest, including BMI and DMFT scores, or would reveal an association with the presence of oral HPV; however, no correlations were found. This may suggest that other variables, which might include the number of previous exposures, parental HPV status, prior protective immunity, socioeconomic status, and even dietary factors, might also influence differences in gender or racial groups who present with HPV.4,5,12,14
As with all biomedical and clinical studies, there were noted limitations in this study. First, due to the nature of all prospective clinical studies dependent upon a convenience sample, selection bias is inherent in the collection of data. Children or parents who might have had previous health issues or other oral problems might be less favorably inclined to participate in a study of this nature. Conversely, children or parents with greater health literacy may be more favorable about participation in these types of studies, which could further influence results. Finally, financial and other limitations of this study prevented collection and analysis of saliva samples that would provide data regarding previous HPV exposure and HPV-protective antibodies, which might have contextualized the understanding of these data in the overall life course of the study participants.
Conclusions
This study provided new evidence that expands the medical and dental fields’ understanding of oral HPV in healthy pediatric patients, particularly within Nevada. More importantly, this study provided findings suggesting that oral HPV may, in fact, be more common than previously described.
About the Authors
Vikram Tiku, DDS
Pediatric Dental Resident
University of Nevada Las Vegas School of Dental Medicine
Las Vegas, Nevada
Chelsie J. Todd
Third-Year Dental Student
University of Nevada Las Vegas School of Dental Medicine
Las Vegas, Nevada
Karl Kingsley, PhD, MPH
Professor
University of Nevada Las Vegas School of Dental Medicine
Las Vegas, Nevada
References
1. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12-19.
2. zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581-587.
3. Shukla S, Bharti AC, Mahata S, et al. Infection of human papillomaviruses in cancers of different human organ sites. Indian J Med Res. 2009;130(3):222-233.
4. Ostwald C, Müller P, Barten M, et al. Human papillomavirus DNA in oral squamous cell carcinomas and normal mucosa. J Oral Pathol Med. 1994;23(5):220-225.
5. Ostwald C, Rutsatz K, Schweder J, et al. Human papillomavirus 6/11, 16 and 18 in oral carcinomas and benign oral lesions. Med Microbiol Immunol. 2003;192(3):145-148.
6. D'Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;53 suppl 1:S5-S11.
7. van Monsjou HS, Balm AJ, van den Brekel MM, Wreesmann VB. Oropharyngeal squamous cell carcinoma: a unique disease on the rise? Oral Oncol. 2010;46(11):780-785.
8. Kingsley K, Johnson D, O’Malley S. Transfection of oral squamous cell carcinoma with human papillomavirus-16 induces proliferative and morphological changes in vitro. Cancer Cell Int. 2006;6:14.
9. Reddout N, Christensen T, Bunnell A, et al. High risk HPV types 18 and 16 are potent modulators of oral squamous cell carcinoma phenotypes in vitro. Infect Agent Cancer. 2007;2:21.
10. Turner DO, Williams-Cocks SJ, Bullen R, et al. High-risk human papillomavirus (HPV) screening and detection in healthy patient saliva samples: a pilot study. BMC Oral Health. 2011;11:28.
11. Flake C, Arafa J, Hall A, et al. Screening and detection of human papillomavirus (HPV) high-risk strains HPV16 and HPV18 in saliva samples from subjects under 18 years old in Nevada: a pilot study. BMC Oral Health. 2012;12:43.
12. Moscicki AB, Puga A, Farhat S, Ma Y. Human papillomavirus infections in nonsexually active perinatally HIV infected children. AIDS Patient Care STDS. 2014;28(2):66-70.
13. Pinheiro RS, de França TR, Rocha B, et al. Human papillomavirus coinfection in the oral cavity of HIV-infected children. J Clin Pathol. 2011;64(12):1083-1087.
14. Mammas IN, Sourvinos G, Giamarelou P, et al. Human papillomavirus in the oral cavity of children and mode of delivery: a retrospective study. Int J STD AIDS. 2012;23(3):185-188.
15. Martinelli M, Zappa A, Bianchi S, et al. Human papillomavirus (HPV) infection and genotype frequency in the oral mucosa of newborns in Milan, Italy. Clin Microbiol Infect. 2012;18(6):E197-E199.
16. Marais DJ, Sampson C, Jeftha A, et al. More men than women make mucosal IgA antibodies to Human papillomavirus type 16 (HPV-16) and HPV-18: a study of oral HPV and oral HPV antibodies in a normal healthy population. BMC Infect Dis. 2006;6:95.
17. Cavenaghi VB, Ghosn EJ, Cruz N, et al. Determination of HPV prevalence in oral/oropharyngeal mucosa samples in a rural district of São Paulo. Braz J Otorhinolaryngol. 2013;79(5):599-602.
18. Catmull J, Row L, Repp MR, et al. Newly identified cariogenic pathogen Scardovia wiggsiae detected by polymerase chain reaction in saliva of teenagers and adults in southern Nevada. Forum for Dental Student Research and Innovation. Spring 2014;2(1):28-35.
19. McOrist AL, Jackson M, Bird AR. A comparison of five methods for extraction of bacterial DNA from human faecal samples. J Microbiol Methods. 2002;50(2):131-139.
20. Inferences about population means. In: Hays WL. Statistics. 5th ed. International Thomson Publishing; 1994:311-342.
21. Jang S, Spader ET, Thacker M, et al. Access to care for pediatric, Medicaid-insured patients in Clark County, Nevada. Journal of Theory and Practice of Dental Public Health. 2013;1(2):37-43.
22. Rice PS, Mant C, Cason J, et al. High prevalence of human papillomavirus type 16 infection among children. J Med Virol. 2000;61(1):70-75.
23. Kojima A, Maeda H, Kurahashi N, et al. Human papillomaviruses in the normal oral cavity of children in Japan. Oral Oncol. 2003;39(8):821-828.