An Evaluation and Comparison of the Efficacy of Nanocrystalline Calcium Sulfate Bone Grafts (NanoGen) and Medical-Grade Calcium Sulfate Bone Grafts (DentoGen) in Human Extraction Sockets
Bindiya Kumari, BDS, MDS; D.K. Gautam, BDS, MDS; Robert A. Horowitz, DDS; Ashish Jain, MDS; and Ajay Mahajan, BDS
ABSTRACT:
Background: Grafting a fresh extraction socket is essential for successful regeneration of bone and maximizing volume preservation. Various synthetic grafts have been used to simulate bone formation. The purpose of the present study was to evaluate clinical, histomorphometric, and radiographic healing at 1-month, 3-month, and 4-month time points after tooth extraction with placement of calcium sulfate hemihydrate putty bone grafts NanoGen and DentoGen to determine their efficacy in ridge preservation following tooth extraction. Method: Sixty subjects who were in need of extraction were recruited. The subjects were randomly assigned their group based on computer software for both the test groups (NanoGen and DentoGen). DentoGen is a medical-grade calcium sulfate hemihydrate with particle of 30 µm, and NanoGen is a nanocrystalline version of DentoGen with particle size 400 µm to 800 µm. Data were recorded at 1, 3, and 4 months after extraction socket grafting. Bone biopsies were taken at 4 months for histomorphometric analysis. Results: The mean percentage of bone formed by NanoGen was 51.19 ± 9.53% and by DentoGen 50.67 ± 16.16% after 4 months. No statistically significant difference was noted in the mean bone formation by NanoGen and DentoGen at various time intervals; no bone graft remnants of DentoGen were found at 4 months. The mean percentage of bone graft remnants left after 4 months for NanoGen was 6.83 ± 16% in the maxilla and 7.38 ± 21% in the mandible. The mean percentage of soft tissue formed was significantly higher with DentoGen in mandibular socket sites. On radiographic evaluation the mean percentage of socket fill with DenoGen was found to be 23.1 ± 11.65%, 50 ± 9.6%, and 76.7 ± 11% and with NanoGen was 29.2 ± 12.8%, 52.8 ± 15.6%, and 76.47 ± 12.43% at 1 month, 3 months, and 4 months postoperative intervals, respectively. Conclusion: Both the materials investigated in the study showed excellent bone forming capacity, but the nanocrystalline version (NanoGen) of calcium sulfate was found to have clinical and biologic advantages over DentoGen.
Introduction
Post-extraction alveolar ridge resorption is an inevitable process. Molar ridges present a higher degree of resorption than premolar areas do.1 Schropp et al reported that the width of the alveolar bone is reduced 50%, and two-thirds of that loss occurs in the first 3 months after extraction.2 The reduction in the height of the bone is more pronounced on the buccal than on the lingual sites.3 The use of invasive techniques is not recommended at this timepoint. Most of the techniques utilized to obtain primary intention healing with the advancement of flaps result in an increased inflammatory response. Simultaneously, there is a decrease in vestibular depth and often the formation of unesthetic scars.1 For these reasons, less invasive grafting techniques might be indicated. Socket grafting techniques have been readily adopted by dentists throughout the world when they can be employed without the elevation of mucoperiosteal flaps, as there is less alveolar bone loss postoperatively.3
DentoGen® (medical-grade calcium sulfate hemihydrate, particle size 30 µm) is a patented nanocomposite bone graft developed by Orthogen, LLC (www.orthogencorp.com) and New York University. Most synthetic and non-synthetic bone graft materials resorb or degrade either too slowly or too quickly; as a result they often do not stimulate adequate bone regeneration. DentoGen degrades completely in 8 to 12 weeks in vivo and releases stimuli for bone growth as it degrades. The basis behind this property is that the rate of resorption of DentoGen matches the physiological bone resorption of the body. This completely matches the time of bone matrix formation and, hence, there is no time lag between the bone matrix formation and bone graft resorption.4
NanoGen® (Orthogen, LLC) is a nanocrystalline version of DentoGen with a particle size ranging from 400 µm to 800 µm. Each particle contains grains of nanocrystalline calcium sulfate (CaSO4) of sizes 200 to 900 nanometers.5 A box of NanoGen also comes with two patient-specific kits. Each kit contains 1 gram of NanoGen. It also comes with saline, which is added to NanoGen drop by drop to transform the material into a putty consistency, making the material moldable and easy to handle. After placement, NanoGen undergoes controlled degradation over 3 to 4 months. This leads to deposition of calcium phosphate, which further stimulates bone formation.6 It is found that the bone graft with particle size of < 60 µm gets phagocytosed in the body. Grafts of particle size < 100 µm cannot promote the capillary ingrowth and bone formation. Grafts of particle size > 300 µm are found to be effective in new bone formation.7
The purpose of this study is to comparatively evaluate the bone forming efficacy of nanocrystalline calcium sulfate hemihydrates (NanoGen) and medical-grade calcium sulfate hemihydrates (DentoGen) in fresh extraction socket sites.
Materials and Methods
The study was conducted in the Department of Periodontology, HP Government Dental College, Shimla, India, from January 1, 2011, to June 30, 2012. Sixty subjects/sites in the age group ranging from 18 to 50 years were divided into two groups of 30 subject/sites each for comparison. Group A included 30 sites/subjects in which extraction sites were treated with nanocrystalline calcium sulfate hemihydrate bone grafts (NanoGen) immediately after local control of bleeding at the extraction sites. Group B included 30 sites/subjects in which extraction sites were treated with medical-grade calcium sulfate hemihydrate bone graft (DentoGen) immediately after local control of bleeding at the extraction sites. Subject selection criteria included systemically healthy subjects who were in need of extractions due to any ongoing pathosis, eg, periapical radiolucency, periapical abscess, etc. Subjects with any of the following conditions were excluded from the study: non-compliant subjects, current smokers, medically compromised subjects, pregnant females, and subjects in whom atraumatic extractions were not possible. The subjects who fulfilled the inclusion criteria were treated utilizing the graft material.
The procedure along with its complications were explained to all the subjects prior to their enrolling in the study, and a written consent was attained. After patient consent, the procedure was done under 2% xylocaine with (1:100,000) adrenaline. The participating subjects were given an intracrevicular incision extending from the mesial to the distal side of the tooth/teeth to be extracted, and a conservative mucoperiosteal flap was raised. Careful tooth/teeth extraction was performed using a periotome. After socket debridement, depending on the group allotted to the subject, granules of either nanocrystalline calcium sulfate bone graft or medical-grade calcium sulfate bone graft material were mixed with normal saline/potassium sulfate (K2SO4) and packed into the defect, filling it to ideal contour immediately after local control of bleeding from extraction. After adequate condensation of the bone grafts, the previously raised flaps around the extraction site were closed with 3-0 silk sutures to secure the bone grafts in place. No osteoprotective regenerative barrier was used, as both the calcium sulfate bone grafts under study have self-retentive properties.
Postoperative care included the use of 0.12% chlorhexidine rinses for 4 weeks, systemic antibiotics for 1 week, and analgesic medication for 3 days; the same regimen was extended as per the need of the subject. Sutures were removed after 10 to 14 days. Clinical photographs were taken at every visit to monitor the soft-tissue response to the graft material. One case in the study encountered complication with swelling of the lower lip and pus discharge; she was given proper medical care and was excluded from the study as a dropout case. Additionally, two patients did not report due to unknown reasons.
Intraoral Periapical (IOPA) Radiographs
Intraoral periapical (IOPA) radiographs were taken at baseline, ie, immediately before the surgical procedure, and at 1-month, 3-month, and 4-month intervals after the surgical procedure to evaluate the initial versus the final radiographic density of bone. The radiographs were taken by paralleling technique with the following parameters: the tube current of 8 mA, the tube voltage of 70 kV, length of the cone (PIG) 12 inches. All of the radiographs were traced on a single sheet of x-ray tracing paper in terms of bone fill.
The preoperative radiograph was used as a template from which the socket outline, the mesial and distal bone crestal levels (of the tooth to be extracted), and a reference line (which passed through the highest point of the adjacent teeth) were marked. Subsequent radiographs at 1 month, 3 months, and 4 months were then superimposed, and bone fill was traced on the same x-ray tracing paper and measured in millimeters from the most apical point towards the coronal point of the socket outline. The lowest level of bone fill was taken into consideration, and the millimeters of bone fill was then converted in terms of percentage socket fill for every month. A rotational panoramic radiography (OPG) was taken preoperatively to measure the height of the alveolar ridge.
Histopathological Evaluation
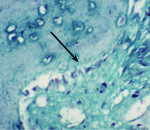
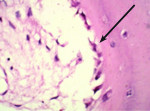
Trephined cylindrical sample cores of newly formed intrasocket tissue, 2 mm in diameter and 7 mm in length, were obtained after 4 months of the surgical procedure. The core was fixed in 10% formalin and then transferred to different gradients of alcohol concentrations (70% ethanol for 24 hours, 95% ethanol for 24 hours, 100% ethanol (x2)(48hrs). After dehydration, the sample was infiltrated and embedded in poly(methyl methacrylate) (PMMA). Sectioning was performed with a low-speed saw microtome. The slide was ground and polished to a thickness of 100 μm and then stained with hematoxylin and eosin, and a few with Masson’s trichome stain (Figure 1) additionally. A slide scanner (Modis™) was used to image the sample, and Image-Pro® Plus (IPWIN) software was used for the histomorphometrical assessment of bone formation. Histomorphometrical analysis was conducted to quantify the amount of total vital bone and soft tissue present in the core. Results were compared in terms of the extent of resorption of both NanoGen and DentoGen, the type of bone formed at histological evaluation, the percentage of bone formed, and the percentage of soft tissue formed.
Results
Demographics
The mean age for test groups was 40.90 ± 19.89 years (range: 18 to 60 years of age). The percentage of males and females of the subjects included in the study was 59.3% male and 40.7% female (sex ratio [male:female] of 59.3:40.7).
Histomorphometry
Residual Socket Fill
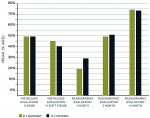
Table 1 and Figure 2 compare the mean histologic percentage of bone formed and histologic percentage of available soft tissue at 4 months postoperatively. Radiographic percentage of socket fill at 1-month, 3-month, and 4-month intervals is also compared for both groups. Group statistical analysis shows non-significant difference between the two groups in terms of bone formation at various time intervals (P > 0.001, ie, 0.870 for % bone, 0.303 for % soft tissue, 0.832, 0.881, and 0.822 for radiographs at various intervals). Table 1 and Figure 2 also show the mean percentage of histologic and radiographic bone formation at various time intervals in maxilla and mandible. The statistical analysis showed non-significant differences in terms of bone formation in the maxilla and mandible (P > 0.005, ie, 0.109, 0.031, 0.730, 0.553, 0.567, respectively).
Bone Graft Remnants
Table 2 outlines the histologic evaluation of mean percentage bone formed, percentage soft tissue present, and percentage of bone graft remnants left after 4 months with the two forms of calcium sulfate (DentoGen and NanoGen) evaluated in this study.
Radiographically
The mean percentage of radiographic socket fill at 4 months with NanoGen was 76.810 ± 13.73% and with DentoGen 74.98 ± 13.44% in mandible and 76.05 ± 11.48% and 77.72 ± 94% in maxilla by NanoGen and DentoGen, respectively.
Discussion
Bone augmentation techniques using calcium sulfate have demonstrated potential for bone regeneration in surgical therapy for more than 100 years.8 Calcium sulfate and calcium phosphate compounds are attractive alternatives to autografts because of their biocompatibility, handling characteristics, porosity, and different rates of dissolution, chemical and physical resemblance to bone mineral, and potentially unlimited supply at a modest cost.9 Originally, calcium sulfate was perceived only as a defect filler, but many recent studies have shown it to be biocompatible, biodegradable, osteoconductive, safe, and non-toxic. It also has angiogenic,5 hemostatic, and barrier membrane properties better than other graft materials. Fibroblast migration over calcium sulfate is maximized compared to other materials.10,11 It can also be combined with other bone graft materials like autografts, allografts, calcium phosphates, and bioactive glasses to increase their binding properties, volume, and effectiveness as bone grafts. Upon implantation in the body, it dissolves into calcium and sulfate ions. Calcium ions combine with phosphate ions from body fluids to form calcium phosphate. This forms an osteoconductive lattice of biologic apatite that stimulates bone growth into the defect. Infrared spectroscopy studies have shown that the newly deposited material is mainly carbonated hydroxyapatite, which is similar to the apatite naturally present in bone.12
In 2009, Horowitz et al discussed the use of DentoGen in combination with beta-tricalcium phosphate (β-TCP) to treat molar extraction sockets and found 32% vital bone and only 8% residual graft in the maxillary sites after its use, but in mandibular molar sites 51% vital bone resulted with less than 1% residual bone replacement graft after 6 months.13 A similar study on NanoGen in 2012 revealed that on clinical, radiographic, and computed tomography (CT) scan inspection at 6 months following grafting, keratinized soft tissue and ideal bone volume was achieved for implant placement. Histomorphometric analysis of a core extracted from the regenerated socket showed 47% vital bone volume with osteoclasts and osteoblasts and remodeling trabecular bone.14 Another similar study in 2012 that comparatively evaluated two forms of calcium sulfate hemihydrate for the treatment of infrabony defects revealed that enhanced gain in defect fill and resolution of osseous defects using nanocrystalline calcium sulfate had a biological and clinical advantage over traditional calcium sulfate, which could be attributed to its slow degradation profile that is closer to the rate of bone growth.15
The healing was uneventful in all the cases, with no clinical signs of inflammation nor local foreign body reaction. Radiographic analysis of healing was performed with a novel approach. The extraction sockets were traced to determine volume initially and at the timepoints studied. On radiographic evaluation the mean percentage socket fill with DentoGen was found to be 23.1 ± 11.65%, 50 ± 9.6%, and 76.7 ± 11%. Sockets that were treated with the nanocrystalline NanoGen had a mean socket fill of 29.2 ± 12.8%, 52.8 ± 15.6%, and 76.47 ± 12.43% at 1-month, 3-month, and 4-month postoperative intervals, respectively.
The 4-month surgical biopsy demonstrated that the augmented socket sites were clinically well preserved in their volume dimensions. Histology examination revealed an abundant amount of new viable bone formation in all the specimens. Close examination of the sections revealed vital bone with a large population of osteocytes and osteoblasts. Between different areas of bone, cellular connective tissue with a large number of fibroblasts was observed. At the periphery of the sections, bone graft remnants surrounded by newly formed bone were identified; most of the bone appeared woven and some was of lamellated type. In contrast, natural healing of extraction socket sites at 4 months presents alveolar trabecular bone up to the crest, with little osteogenesis and only occasional osteoblasts.16
Two parallel series of mechanisms are triggered by the degradation of calcium sulfate in a deep bone defect. The first mechanism involves the release of calcium and sulfur ions into the biological environment, which results in carbonate apatite formation and calcium ion stimulation of cellular activity.17 The second mechanism is the precipitation of calcium phosphate, which leads to a transient, local drop in pH. This causes surface demineralization of existing bone, resulting in exposure of bioactive molecules and the local release of growth factors. Transforming growth factors and bone morphogenetic proteins enter the site and stimulate the growth of bone in defects filled with calcium sulfate.18 Calcium sulfate has been used as a bone graft by itself, in combination with other bone grafts, and also as a barrier to treat extraction sockets.19
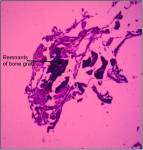
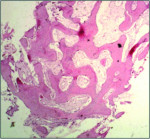
The mean percentage of bone formed with NanoGen (Figure 3) in the study after 4 months was 51.19 ± 9.53% and with DentoGen (Figure 4) 50.67 ± 16.16%. There was no statistically significant difference in bone formation by NanoGen and DentoGen at various time intervals. The mean volume of bone graft remnants left after 4 months in the histologic reports of NanoGen slides was 12.35%. There was no difference in the percentage bone formation in maxilla or mandible with the same bone graft material. The mean bone formation in the mandible with DentoGen was 45.20%, and with NanoGen 50.33%; the mean bone formation in the maxilla with Dentogen was 54.150% and with Nanogen 52.26%. The percentage of soft tissue formed was significantly higher with DentoGen in the mandibular socket sites. The presence of NanoGen particles at 4 months in all histologic specimens shows that the resorption rate of these particles is extremely slow. The manufacturer of the product claims that the unique crystalline nature of the NanoGen calcium sulfate prevents the resorption of these particles. The bone formed was in an advanced state of maturation with osteoblasts lining the surface of the new bone (Figure 5), showing that continued bone formation was occurring.
Conclusion
Extraction site collapse is a problem for patients that has physical, biologic, and esthetic consequences. There are numerous bone replacement graft materials and barriers utilized worldwide in an attempt to counter this issue. Use of calcium sulfate-based products offers multiple advantages. With more than a century of research, these materials are based highly on science. According to this study (and others on calcium sulfate), no barrier was needed over the socket replacement bone graft. With no to minimal residual graft, there is less of a chance of interference with the process of osseointegration than might occur with non- or slowly resorbing biomaterials placed in extraction sockets. As the materials are inserted and seen as a radiopaque mass and then become radiolucent in 2 to 4 weeks, the surgeon and restorative dentist can follow healing very easily with simple periapical radiographs. The use of only one biomaterial enabled this process to be less expensive for the dentist and patient while giving a very high predictability of obtaining the ideal results the surgeon is interested in: minimal residual graft, a high degree of bone volume preservation, and formation of a large percentage of vital bone. With more than 50% vital bone formed (Figure 6) and minimal residual graft material, sites like this should be suitable for earlier prosthetic loading, enabling patients to return to esthetics and function at a faster rate.
References
1. Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biologic basis and treatments. Int J Dent. 2012; Article ID 151030, 13 pages. doi:10.1155/2012/151030. https://www.hindawi.com/journals/ijd/2012/151030/. Accessed October 3, 2014.
2. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
3. Fickl S, Zuhr O, Wachtel H, et al. Tissue alterations after tooth extraction with and without surgical trauma: a volumetric study in the beagle dog. J Clin Periodontol. 2008:35(4):356-363.
4. DentoGen. Orthogen, LLC website. www.orthogencorp.com/our_product/dentogen.php. Accessed October 3, 2014.
5. Kaigler D, Pagni G, Park CH, et al. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A. 2010;16(9):2809-2820.
6. Zaner DJ, Yukna RA. Particle size of periodontal bone grafting materials. J Periodontol. 1984;55(7):406-409.
7. Shapoff CA, Bowers GM, Levy B, et al. The effect of particle size on the osteogenic activity of composite grafts of allogeneic freeze-dried bone and autogenous marrow. J Periodontol. 1980;51(11):625-630.
8. Maragos P, Bissada NF, Wang R, Cole RP. Comparison of three methods using calcium sulfate as a graft barrier material for the treatment of Class II mandibular molar furcation defects. Int J Periodontics Restorative Dent. 2002;22(5):493-501.
9. Sopyan I, Mel M, Ramesh S, Khalid KA. Porous hydroxyapatite for artificial bone applications. Sci Technol Adv Mater. 2007;8(1-2):116-123.
10. Payne JM, Cobb CM, Rapley JW, et al. Migration of human gingival fibroblasts over guided tissue regeneration barrier materials. J Periodontal. 1996;67(3):236-244.
11. Aichelmann-Reidy ME, Heath CD, Reynolds MA. Clinical evaluation of calcium sulfate in combination with demineralized freeze-dried bone allograft for the treatment of human intraosseous defects. J Periodontol. 2004;75(3):340-347.
12. Anson D. Saving periodontally ‘‘hopeless teeth’’ using calcium sulfate and demineralized freeze-dried bone allograft. Compend Contin Educ Dent. 1998;19(3):284-288.
13. Horowitz RA, Rohrer MD, Prasad HS, Mazor Z. Enhancing extraction socket therapy. Journal of Implant & Advanced Clinical Dentistry. 2009:1(6);47-59.
14. Mazor Z, Horowitz RA, Ricci J, et al. The use of a novel nano-crystalline calcium sulfate for bone regeneration in extraction socket. Journal of Implant & Advanced Clinical Dentistry. 2011;3(5):39-49.
15. Rouble K, Pandit N, Ashish J, et al. Comparative evaluation of two forms of calcium sulfate hemihydrate for the treatment of infrabony defects. Indian Journal of Dental Sciences. 2012;4(2)30-36.
16. Steiner GG, Francis W, Burrell R, et al. The healing socket and socket regeneration. Compend Contin Educ Dent. 2008;29(2):114-124.
17. Ricci JL, Alexander H, Nadkarni P, et al. Biological mechanisms of calcium sulfate replacement by bone. In: Davies JE, ed. Bone Engineering. Toronto, Canada: em squared Inc.; 2000:332-344.
18. Walsh WR, Morberg P, Yu Y, et al. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res. 2003;(406)228-236.
19. Mazor Z, Mamidwar S, Ricci JL, Tovar NM. Bone repair in periodontal defect using a composite of allograft and calcium sulfate (DentoGen) and a calcium sulfate barrier. J Oral Implantol. 2011;37(2):287-292.
About the Authors
Bindiya Kumari, BDS, MDS
Senior Resident – Periodontics, CDER, AIIMS (All India Institute for Medical Sciences)
Delhi, India
D.K. Gautam, BDS, MDS
Professor and Head, Department of Periodontology
Himachal Dental College
Sundernagar, India
Robert A. Horowitz, DDS
Clinical Assistant Professor
Departments of Implant Dentistry and Periodontics
Oral Surgery
New York University College of Dentistry
New York, New York, USA
Ashish Jain, MDS
Principal, Professor and Head
Department of Periodontology
H.S.J. Institute of Medical Sciences
Panjab University
Chandigarh, India
Ajay Mahajan, BDS
Assistant Professor, Department of Periodontology and Implantology
HP Government Dental College
Shimla, India