Treating Peri-Implantitis Using a Combined Regenerative/Resective Procedure: A Case Report
Abstract
The aim of this article is to describe a combined regenerative/resective procedure used in the treatment of peri-implantitis. The following case presented with excess-cement-induced peri-implantitis and the resulting combined circumferential and buccal dehiscence defect. Surgical therapy consisted of minimal resection of interproximal peaks of bone and subsequent guided bone regeneration. The 12-month clinical result was resolution of peri-implantitis (no pathologic probing depths, bleeding on probing, suppuration), radiographic evidence of bone fill of the defect, and some recession that was clinically acceptable to the patient.
One of the common biological complications associated with dental implants is peri-implantitis. Peri-implantitis has been defined as an inflammatory process affecting the tissues around an osseointegrated implant in function, resulting in the loss of supporting bone.1 Progression of such bone loss may lead to eventual implant failure.
Peri-implantitis may occur due to various causative agents, including poor restoration seating, implant malposition, technical complications, traumatic placement, and unretrieved excess cement after restoring implants.2 Wilson showed that excess dental cement was associated with signs of peri-implantitis, and, upon removal, signs of peri-implantitis resolved in 74% of test implants.3 Wadhwani showed that many cements commonly used for the cementation of implant-supported prostheses are undetectable radiographically.4
The incidence of chronic inflammation of soft and hard tissues neighboring implants after 5 years has recently been reported to range from 8.6% to 9.7% and can develop within months of implant placement or years thereafter.5,6 Currently used treatment modalities include alterations to microflora, as well as resective and regenerative techniques. Antimicrobial therapies have recently been shown to produce unpredictable and unmaintainable results.7 Surgical techniques, including guided bone regeneration (GBR), have been shown to be more predictable in correcting peri-implant defects; however, there is debate about whether this grafted bone osseointegrates into the affected implant surface.8
The ultimate goals of such therapies include the reduction of bacterial load and re-establishment of clinical peri-implant health. Recent findings indicate that the nature of the bone defect around a fixture afflicted with peri-implantitis can have a significant impact on the expected outcome of treatment.9
Various techniques have been employed to treat periodontal diseases associated with attachment loss, including both surgical and nonsurgical approaches. Of these techniques, osseous resection is often considered a “compromised” approach in that it reduces pocket depth, but at the cost of supporting bone. In Ochsenbein’s words, “The primary objective of osseous surgery is to remove the minimal amount of bone that will meet the needs of an adequate architectural form.”10 While it is now generally accepted that regaining attachment through the use of grafting and/or growth factors is preferable, in some situations, osseous resection is still an effective method of reducing pocket depth. A recent study involving resection of peri-implantitis defects showed complete resolution of peri-implantitis symptoms in 58% of cases after 2 years.11
This case presentation demonstrates the use of GBR in combination with selective resection to repair an osseous defect resulting from peri-implantitis.
Case Presentation
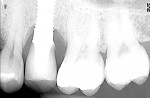
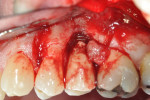
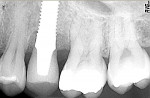
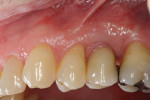
A 40-year-old man with a non-contributory medical history presented with a chief complaint of discomfort, discoloration, and bleeding on brushing around his restored implant at the No. 13 position. The patient reported that the implant had been placed 18 months prior and was subsequently restored with a single-unit, cement-retained, porcelain-fused-to-metal (PFM) restoration. Radiographs revealed bone loss in a pattern suggesting peri-implantitis (Figure 1). Probing depths ranged from 8 mm to 10 mm, with moderate bleeding on probing and suppuration (Figure 2).
A discussion with the patient regarding the elimination of his peri-implantitis issue ensued. The patient was told that several techniques could be employed to treat his condition, and that surgical intervention with grafting is usually more effective in reversing the bone loss he had already experienced. The alternative options of implant removal with subsequent grafting and placement of another implant in the future, pure resective therapy, and non-surgical intervention were all presented and rejected by the patient. The patient was aware that recession and loss of attachment to adjacent teeth was possible with any surgical intervention. Finally, the possibility of removing the implant crown in order to possibly obtain a better regenerative result while submerging the implant/graft was presented to the patient. The patient declined this option because the cost of fabricating a new prosthesis was financially burdensome for him.
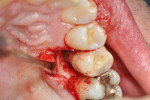
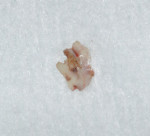
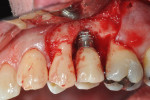
After discussing the risks and benefits of surgery with the patient, the decision was made to re-enter the No. 13 site. The patient was anesthetized with local infiltration of 2% lidocaine with 1:100,000 epinephrine. Incisions were made in a sulcular fashion on the buccal and palatal of Nos. 12 through 14 in order to preserve as much soft tissue as possible, with buccal vertical releases at the mesial of Nos. 14 and 12. Surgically exposing the coronal portion of the implant with full-thickness buccal and palatal flaps and removal of interproximal tissue revealed a circumferential osseous defect with a buccal dehiscence (Figure 3 and Figure 4) surrounding a piece of excess cement attached to the buccal portion of the implant–crown interface (Figure 3).
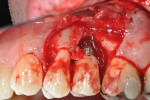
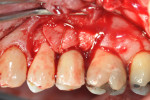
Treatment of the area began with mechanical removal of the cement and thorough removal of all soft tissue from the osseous defects using hand instruments (Figure 5 and Figure 6). Minimal resection of the osseous peaks on the mesial of No. 14 and the distal of No. 12 was performed. This ostectomy was performed in order to create a shallower osseous defect surrounding the implant. While repair and bone fill often favors narrow and deep defects, these defects often do not fill completely.12-14 Subsequent procedures are sometimes required to either attempt further repair or fill the defect with more bone or to resect the area to eliminate the remaining defect.15 Therefore, this procedure attempted to create a defect that may achieve a reasonable amount of bone fill via grafting while not requiring a secondary resection procedure.
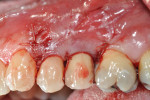
Additionally, the buccal portion of the implant threads were removed/reduced using a high-speed handpiece (Figure 7) in an attempt to position the buccal outline of the implant further within the alveolar housing, thereby increasing the potential success of the regenerative therapy. The elimination of implant threads has been used as a treatment modality for peri-implantitis cases in the past with successful results (ie, clinically “healthy pink appearance” and radiographically “absence of pathosis”) reported.16,17 While there is currently no literature explaining how the resection of implant threads will affect osseointegration, it can be assumed that the thread removal will simultaneously remove any endotoxins and other factors that may negatively affect future bone fill and/or osseointegration. Finally, the removal of implant threads creates additional space for grafting material, which can itself offer space maintenance and clot stabilization to promote filling of the defect. This also served an additional purpose of removing portions of the contaminated rough implant. Debris from the removed treads was collected using high-speed suction during resection and subsequent irrigation using sterile saline. Implant and root conditioning was then performed using a tetracycline-saline slurry applied topically and scrubbed for 5 minutes. Guided bone regeneration was then employed, using an inner layer of freeze-dried bone allograft (FDBA) that is amenable to turnover and replacement by host tissues, and an outer layer of bovine-derived hydroxyapatite (Bio-Oss®, Geistlich Biomaterials, www.geistlichonline.com) that is relatively resistant to turnover. The grafting materials were then covered on the buccal, mesial, distal, and palatal aspects with a resorbable collagen membrane (Ossix™ Plus™, OraPharma, Inc., www.orapharma.com), which was secured into place using a horizontal mattress suture of 5-0 chromic gut material (Figure 8).
Primary tension-free closure was achieved and sutured into place using single interrupted 5-0 chromic gut material (Figure 9). An immediate postoperative radiograph showed grafting material at the level of the new interproximal peaks of bone of adjacent teeth (Figure 10). Postoperative prescriptions included chlorhexidine 0.12% rinse twice daily for 2 weeks, amoxicillin 500 mg every 8 hours for 10 days, and ibuprofen 800 mg every 6 to 8 hours as needed for pain. The patient was instructed not to brush the surgical site or adjacent teeth for 2 weeks and not to floss the site for 4 months following the procedure.
Results
At the 1-week, 3-week, 3-month, and 6-month postoperative visits, the patient received a localized professional cleaning consisting of removal of plaque and calculus using hand instruments and Cavitron® (DENTSPLY Professional, www.dentsply.com). At these visits, the patient exhibited uneventful healing (progressive decline in erythema and edema associated with normal healing and lack of suppuration) of the surgical site.
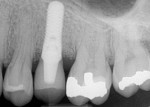
Six months after the corrective surgery, a clinical examination was performed and radiographs were obtained. Examination of the area revealed 1-mm to 2-mm recession along the buccal surface of the implant and adjacent crowns. Probing depths ranged from 2 mm to 3 mm at the implant site and adjacent teeth with no bleeding on probing and no suppuration. Radiographs suggested bone fill of the circumferential osseous defect. A clinical examination (Figure 11) and radiograph (Figure 12) at 12 months showed that the defect maintained this disease-free state and radiographic bone levels.
Discussion
Conservative approaches to treating peri-implantitis include scaling of the affected dental implant with and without irrigation substances and/or antibiotics.18,19 These conservative measures are minimally invasive and have been shown to result in the improved outcomes of clinical attachment gain (CAL) and pocket probing depth (PPD) reduction. However, there is limited evidence that surgical procedures with and without bone regeneration promote better PPD and CAL changes.20,21 While many different techniques comprise both the nonsurgical and surgical modalities of treating peri-implantitis, a recent network meta-analysis showed that surgical techniques using GBR achieved 3.52 mm greater PPD reduction and 2.80 mm greater CAL gain than nonsurgical therapy alone.22
Buser showed that the limitations of GBR are confined to the interproximal peaks of bone of adjacent teeth and that horizontal augmentation is relatively predictable, while vertical augmentation can be quite challenging.23 Grunder showed that the location of the interproximal peaks of bone on natural teeth adjacent to implants determine the anticipated quality of interproximal soft tissues.24 It has also been shown that having an adequate implant surface to outer crest of bone dimension at the time of immediate implant placement is necessary to maintain an esthetic and functional amount of bone on the buccal aspect of implants.25,26
It is therefore not surprising that the nature of the osseous defect surrounding a fixture afflicted with peri-implantitis may have a significant impact on the ability to treat it surgically. Specifically, circumferential osseous defects without a buccal dehiscence responded to GBR with higher changes in PPD and CAL compared to osseous defects with a buccal dehiscence component.9
These reports support the idea that placing an implant within the alveolar housing is an important factor in maintaining buccal bone. Therefore, if an implant exhibits buccal and circumferential bone loss consistent with peri-implantitis, it makes sense that GBR of this defect should be more predictable and successful for an implant originally placed within the alveolar housing. Furthermore, in the case of a buccal defect due to peri-implantitis, the resection of the buccal threads of the affected implant further contains the implant within the alveolar housing and should improve the predictability of a regenerative procedure.
Past regenerative approaches for treating peri-implantitis defects using GBR have included the use of bovine bone,27 other bone grafts, and bone substitutes.28,29 These studies have reported favorable results by reducing probing depths from 2.1 mm to 5.4 mm, filling of the defects from 2.3 mm to 3.75 mm, and decreasing bleeding on probing after 1 to 7.5 years.
Periodontal literature is rife with techniques used to treat periodontal diseases associated with attachment loss around teeth. Osseous resection is often considered a “compromised” technique in that while it effectively reduces pocket depth, it does so at the cost of supporting bone. Generally speaking, regaining attachment through the use of grafting is preferable to osseous resection; however, many osseous defects are not amenable to regeneration or repair. Therefore, osseous resection still has a place in treating certain osseous defects and reducing pocket depth. Serino and Turri applied a resective therapy (without GBR) to peri-implantitis sites but were completely successful in resolving the signs of peri-implantitis in only 58% of cases after 2 years.11
The current case report illustrates the treatment of peri-implantitis resulting in a circumferential osseous defect with a buccal dehiscence. This combination defect arose within 18 months of the accidental application of excess restorative cement. Upon entering the clinical site and removal of the offending cement, the decision was made to resect a portion of the interproximal peaks of bone and then graft the resulting defect using a standard GBR technique. By resecting the interproximal peaks of bone, a partially compromised outcome that involved some recession along with a reduction in pocket depth was anticipated. The resulting defect was also transformed into one that was more amenable to a successful GBR outcome.
The 6-month results of this combination procedure yielded a PPD reduction of 6 mm to 7 mm, which included a CAL gain of 5 mm and 1 mm to 2 mm of recession along the buccal surface of the implant; 1 mm to 2 mm of recession was also encountered on the buccal surfaces of the adjacent crowns. Both the implant crown and adjacent teeth exhibited no bleeding on probing and no suppuration 12 months post-surgically. Twelve-month radiographs suggested bone fill of the combined circumferential/buccal osseous defect.
Due to the recession commonly encountered with resective techniques, the use of such procedures is often limited to non-esthetic areas of the mouth. Therefore, this procedure should be used with caution in areas where esthetics is of paramount importance.
GBR has been shown to be a viable treatment modality for peri-implantitis in some situations. However, when combined with a resective procedure, a more significant reduction in probing depth is possible. However, this added benefit comes at the expense of additional recession. The decision to pursue this combination technique lies in a joint decision between patient and clinician after all risks, benefits, and alternatives have been explained.
Conclusion
The results from this study suggest that peri-implantitis as a result of excess restorative cement can be resolved by a combination of regenerative (GBR using FDBA, Bio-Oss, and resorbable Bio-Gide membrane) and resective (removing osseous peaks from adjacent teeth) procedures. Although the authors’ results appear to have achieved a state of health clinically, the 1 mm to 2 mm of recession experienced was esthetically acceptable to the patient.
References
1. Albrektsson T, Isidor F. Consensus report of session IV. In: Lang NP, Karring T, eds. Proceedings of the First European Workshop on Periodontology. London, UK: Quintessence Publishing Co. Inc.; 1994:365-369.
2. Lang NP, Berglundh T. Periimplant diseases: where are we now?—Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(suppl 11):178-181.
3. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392.
4. Wadhwani C, Hess T, Faber T, et al. A descriptive study of the radiographic density of implant restorative cements. J Prosthet Dent. 2010;103(5):295-302.
5. Pjetursson BE, Tan K, Lang NP, et al. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin Oral Implants Res. 2004;15(6):625-642.
6. Jung RE, Pjetursson BE, Glauser R, et al. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res. 2008;19(2):119-130.
7. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36(7):604-609.
8. Schwarz F, Sahm N, Bieling K, Becker J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four-year clinical follow-up report. J Clin Periodontol. 2009;36(9):807-814.
9. Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical -outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol. 2010;37(5):449-455.
10. Ochsenbein C. Current status of osseous surgery. J Periodontol. 1977;48(9):577-586.
11. Serino G, Turri A. Outcome of surgical treatment of peri-implantitis: results from a 2-year prospective clinical study in humans. Clin Oral Implants Res. 2011;22(11):1214-1220.
12. Garrett S, Loos B, Chamberlain D, Egelberg J. Treatment of intraosseous periodontal defects with a combined therapy of citric acid conditioning, bone grafting, and placement of collagenous membranes. J Clin Periodontol. 1998;15(6):383-389.
13. Tonetti MS, Pini-Prato G, Cortellini P. Effect of cigarette smoking on peridontal healing following GTR in infrabony defects. A preliminary retrsopective study. J Clin Periodontol. 1995;22(3):229-234.
14. Tonetti MS, Pini-Prato G, Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodonol. 1996;23(6):548-556.
15. Rosenberg MM. Reentry of an osseous defect treated by a bone implant after a long duration. J Periodontol. 1971;42(6):360-363.
16. Lozada JL, James RA, Boskovic M, et al. Surgical repair of peri-implant defects. J Oral Implantol. 1990:16(1):42-46.
17. Kwan JY, Zablotsky MH. Periimplantitis, the ailing implant. Implant Soc. 1991;2(1):6-9.
18. Büchter A, Meyer U, Kruse-Lösler B, et al. Sustained release of doxycycline for the treatment of peri-implantitis: randomised controlled trial. Br J Oral Maxillofac Surg. 2004;42(5):439-444.
19. Schwarz F, Sculean A, Rothamel D, et al. Clinical evaluation of an Er:YAG laser for nonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Implants Res. 2005;16(1):44-52.
20. Romeo E, Ghisolfi M, Murgolo N, et al. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: clinical outcome. Clin Oral Implants Res. 2005;16(1):9-18.
21. Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a prospective cohort study. J Clin Periodontol. 2007;34(7):625-632.
22. Faggion CM Jr, Chambrone L, Listl S, Tu YK. Network Meta-Analysis for Evaluating Interventions in Implant Dentistry: The Case of Peri-Implantitis Treatment. Clin Implant Dent Relat Res. 2011. Aug 11. doi: 10.1111/j.1708-8208.2011.00384.x. [Epub ahead of print]
23. Buser D, Dahlin C, Schenk RK. Guided Bone Regeneration in Implant Dentistry. Chicago, IL: Quintessence; 1994.
24. Grunder U. Stability of the mucosal topography around single-tooth implants and adjacent teeth: 1-year results. Int J Periodontics Restorative Dent. 2000;20(1):11-17.
25. Spray JR, Black CG, Morris HF, Ochi S. The influence of bone thickness on facial marginal bone response: stage 1 placement through stage 2 uncovering. Ann Periodontol. 2000;5(1):119-128.
26. Tomasi C, Sanz M, Cecchinato D, et al. Bone dimensional variations at implants placed in fresh extraction sockets: a multilevel multivariate analysis. Clin Oral Implants Res. 2010;21(1):30-36.
27. Roccuzzo M, Bonino F, Bonino L, Dalmasso P. Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: comparative results of a prospective study on two different implant surfaces. J Clin Periodontol. 2011;38(8):738-745.
28. Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Submerged healing following surgical treatment of peri-implantitis: a case series. J Clin Periodontol. 2007;34(8):723-727.
29. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.
About the Authors
Brock Lorenz Resident
Department of Periodontology
Nova Southeastern University
Fort Lauderdale, Florida
Taeheon Kang, DDS, MS
Private Practice in Periodontics
Fairfax, Virginia