Enhancing Extraction Socket Therapy with a Biphasic Calcium Sulfate
Abstract
Studies have shown that tooth extraction results in loss of bone volume, which compromises dental implant placement. Prevention of site collapse at the time of extraction is recommended. In this 4-month case series, 40 patients were treated with an innovative biphasic calcium sulfate graft, demonstrating its ability to preserve or augment socket volume and resorb in the time period desired between extraction and implant placement. Some representative samples were retrieved at the time of implant placement and evaluated histologically and morphometrically for vital bone formation.
Clinical studies have shown significant bone resorption and volume loss in the first 6 months after tooth extraction.1 Socket augmentation has been advocated to eliminate the need for a secondary reconstructive procedure.2 Many types of graft materials have been examined to prevent this phenomenon, including freeze-dried bone allograft (FDBA),1 anorganic bovine bone mineral (ABBM),3 demineralized freeze-dried bone allograft (DFDBA),4,5 alloplastic materials,6 and mixtures of allograft materials with calcium sulfate.7-9 Studies have also been conducted using only dense polytetrafluoroethylene (PTFE) barriers to protect a blood clot in the extraction socket, thus enabling vital bone formation in the site.10-12
In the present case series, a biphasic calcium sulfate (BCS) was used with no other graft materials mixed in. BondBone™ (MIS Implant Technologies, www.misimplants.com) is composed of two phases of highly pure medical-grade calcium sulfate hemihydrate and dihydrate in a uniquely controlled particle size distribution (Figure 1). The setting time allows the practitioner a reasonable amount of working time of approximately 3 minutes. The heat released after mixing reaches an average reaction temperature of 30°C (85°F) after about 3 minutes, while the pH of the surrounding tissue remains neutral. The inherent dihydrate fraction of this graft mixture reduces the exothermic reaction found in products that use accelerators during setting. This results in reduced patient discomfort.
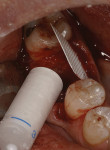
In bone regenerative techniques, BondBone can be used alone, mixed with other suitable bone filling agents to prevent particle migration in an osseous defect or to provide a resorbable barrier over the bone graft material. It has an applicator (Figure 2), which is particularly useful for preparing and applying the bone graft composition while facilitating the procedure. Once the mixture encounters saline, the granulated powder goes through an efficient setting process. This setting allows the in situ formation of a rigid structure, which is highly crystalline despite the intervening harsh environment of blood, proteins, and saliva (Figure 3). The interaction of the two calcium sulfate phases—hemihydrate and dihydrate—shorten the setting time (cement reaction), while the unique particle-size distribution controls the reaction rate, thus determining both the setting time and the microstructure being built.
When tooth extraction is planned, the surgeon should diagnose the alveolar and gingival defects that may be present. Since ideal implant-supported restorations require maximum bone and keratinized tissue, preservation and/or augmentation of these at the time of extraction is the goal of the reconstruction procedure. Achieving this goal with a minimal number of materials and surgical interventions is desirable. The ultimate desired outcome—ie, vital bone formation—maximizes surface contact of the implant with bone cells, which enables osseointegration. Other graft materials utilized in these procedures have met some of these goals. A variety of grafts have preserved socket volume when covered by a barrier or membrane.13 However, histologically, some of these materials did not resorb during the studied time interval,14 resulting in a histologic appearance different from native alveolar bone. Some of these studies have concluded that xenografts are nonresorbable.14,15
The purpose of this study was to evaluate an innovative biphasic calcium sulfate to be used as a graft material at the time of tooth extraction. The ability to preserve or augment socket volume and resorb in the time period desired between extraction and implant placement was analyzed. Additionally, samples were evaluated histologically and using micro CT scan technology for vital bone formation.
Materials and Methods
The study included 40 patients who presented to private dental offices for tooth extraction. A total of 60 teeth were extracted and grafted with BCS. All patients were in good health and had no medical contraindications that would prevent routine dentoalveolar surgery. Preoperative photographs and periapical radiographs were taken of the sites. After administering local anesthesia, teeth were extracted in an atraumatic manner using periotomes and luxators, as well as ultrasonic bone surgical instruments when indicated. Sites were debrided by mechanical means to remove granulation tissue. If the socket was intact, the material was placed without a membrane or barrier. In cases where a dehiscence was present, a resorbable collagen barrier (Cytoplast® RTM, Osteogenics Biomedical, www.osteogenics.com) was placed outside the defect in a subperiosteal manner. In most cases, primary closure was not obtained over the graft material and barrier when placed.
The graft material is packaged in a sterile syringe. BCS powder is wetted with sterile saline before being placed in the socket. Excess liquid was expressed into sterile gauze and the material was injected into the site. After the site was filled to ideal contour, dry gauze was applied and lightly compressed on top of the material. The working time was approximately 2 minutes. In most patients, the set coronal portion of the graft functioned as a barrier. Sites where large amounts of the set calcium sulfate remained exposed to the oral environment were protected with a removable dense PTFE barrier (Cytoplast TXT, Osteogenics) (Figure 2). At 3 weeks postoperatively, the PTFE barrier was removed, leaving the epithelium to keratinize over the osteoid in the socket during the next healing phase.
Patients were monitored for soft-tissue healing and radiographic evidence of graft resorption and bone formation for 3 to 5 months. In some sites, histological specimens were obtained with the use of a trephine drill at the initial osteotomy preparation. All implants placed in these sites were clinically successful.
Histologic Preparation and Histomorphometry
At the time of implant placement, bone cores were harvested from the surgical sites. Trephines containing the bone were fixed in 10% neutral buffered formalin. Upon receipt in the Hard Tissue Research Laboratory at the University of Minnesota Dental School, the specimens were immediately dehydrated with a graded series of alcohols for 9 days. Following dehydration, the specimens were infiltrated with a light-curing embedding resin (Technovit 7200 VLC, Heraeus Kulzer, www.kulzer-technik.de). Following 20 days of infiltration with constant shaking at normal atmospheric pressure, the specimens were embedded and polymerized by 450 nm light with the temperature of the specimens never exceeding 40°C. The specimens were then prepared by the cutting/grinding method of Donath.16,17 The specimens were cut to a thickness of 150 µm on an EXAKT Cutting/Grinding System (EXAKT Technologies, www.exaktusa.com). Slides were then polished to a thickness of 45 µm using the EXAKT microgrinding system, followed by alumina polishing paste and stained with Stevenel’s blue and Van Gieson’s picro fuchsin. Following histologic preparation, the cores were evaluated morphometrically. All the cores were digitized at the same magnification using a Zeiss Axiolab microscope (Zeiss, www.meditec.zeiss.com) and a Nikon Coolpix 4500 digital camera (Nikon, www.nikonusa.com). Histomorphometric measurements were completed using a combination of Adobe® Photoshop® and the public domain NIH Image program (developed at the US National Institutes of Health and available on the internet at https://rsb.info.nih.gov/nih-image/). At least two slides of each core were evaluated. Parameters evaluated were total area of the core, percentage of new bone formation, and percentage of residual graft material.
MICRO CT Preparation and Analysis
Bone cores (stored in 10% formalin) were analyzed using a micro CT (mCT 40, Scanco Medical, www.scanco.ch) with a medium scan mode at an integration time of 200 milliseconds. The total scan time per sample was 26 minutes within the defined volume of interest (VOI). Samples were placed in the micro CT specimen’s 12.3-mm holder and sealed to prevent bones from drying. Scans were performed using medium-resolution (12 μm nominal resolution) to assess the newly formed mineralized structure. Data were collected at 55 kVp and 145 µA, and reconstructed images were filtered using a constrained 3-dimensional (3-D) Gaussian filter to partially suppress noise in the volumes (σ = 1.2 and support = 1), and binarized using a threshold of 322 for patient 1 and 211 for patient 3.
Results
Three of the 40 patients using BCS who had bone removed at the time of implant placement are presented. These were the patients where sites were ideal enough to enable the use of a wide diameter (3.5-mm outer diameter) trephine as the first bur in implant site preparation. These sites were representative of the density and coronal fill of the other sockets treated with biphasic calcium sulfate.
Patient 1
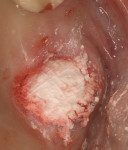
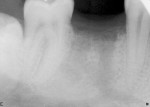
A 79-year-old woman presented with a failing left maxillary second molar tooth under a fixed prosthesis. After bridge removal, the tooth was deemed hopeless. It was sectioned and extracted (Figure 4). The site was thoroughly debrided and grafted to the level of the gingival margin with BCS (Figure 5). No barrier was utilized over the graft material, which healed uneventfully. Two months later, a lateral-approach sinus augmentation was performed. After allowing the sinus graft to heal for 6 months, full-thickness mucoperiosteal flaps were elevated in preparation for implant placement. A trephine was used as the first bur for osteotomy preparation in the left maxillary second molar site. Two dental implants were placed in dense bone and were fully stable at the time of placement. Micro CT examination of the retrieved bone showed vital bone in the site (Figure 6), which was allowed to heal for 6 months prior to uncovering (Figure 7), prosthetic loading, and restoration.
Patient 2
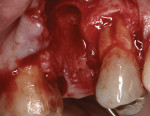
A 44-year-old male patient presented with failed endodontic therapy on the mandibular right first molar. There was a buccal abscess and fistula present when the tooth was extracted, leaving large bony defects on the buccal aspect (Figure 8). The site was fully debrided and grafted with BCS. To assist in containment and bone regeneration, a dense PTFE barrier (Cytoplast TXT) was placed coronally (Figure 9) and over the bony defect on the facial surface to assist in containment and bone regeneration. Primary closure was not obtained. The dPTFE barrier was removed 3 weeks after placement.
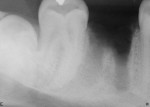
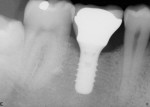
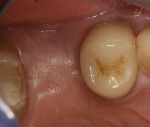
Based on radiographic interpretation, the graft material had resorbed 2 months postsurgery. At 4 months postoperatively, the resorbed graft material had been replaced by a mineralized, trabeculated appearance on radiograph (Figure 10). At this time, the patient was administered local anesthesia. Full-thickness facial and lingual flaps were elevated through the thick, fully healed keratinized tissue. Clinically, the alveolar ridge appeared fully healed. There was no evidence of graft particles. Upon drilling the osteotomy, a core of regenerated bone was harvested. Histologic analysis was performed on the core after non-demineralized processing, which revealed no residual graft material and advanced woven bone formation (Figure 11). The bone was dense, and a wide-neck, one-stage dental implant was inserted and fully stabilized clinically. The implant was restored 3 months after placement with a solid abutment and a ceramometal crown (Figure 12).
Patient 3
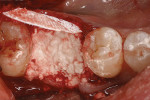
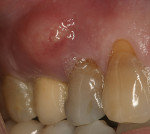
A 57-year-old man presented with failed endodontic therapy on the maxillary right second premolar. The presence of a buccal abscess and fistula (Figure 13) led to a complete loss of the buccal plate. After tooth extraction and flap elevation, the socket was debrided of all remnants of granulation tissue (Figure 14). The socket was grafted with BCS (Figure 15) and covered with a long-lasting resorbable collagen membrane (Osseoguard®, Biomet 3i, www.biomet3i.com).
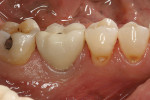
Clinically, the alveolar ridge appeared fully healed 4 months postoperatively (Figure 16). After administering local anesthesia, full-thickness facial and lingual flaps were elevated. The exposed site revealed total reconstruction of the ridge form. A bone core was taken for histological evaluation and micro CT analysis. The micro CT analysis (Figure 17) demonstrated full vital bone reformation in the surgical site. At that time, an appropriate size and shape root-form tapered implant was inserted and restored 5 months later with a custom abutment and ceramometal crown.
Discussion
Technological advances over the past decade have led to a new era in bone repair. Today, augmentation procedures are a part of routine dental surgical care. Current enhancement methods use materials from different sources, classified as autografts, allografts, xenografts, and alloplasts, which are in the form of granules, putties, syringeable gels/pastes, or blocks. The material studied in this report, BondBone™, is unique in that it is a biphasic calcium sulfate that is prepackaged in a syringe and has been developed to facilitate handling and reduce time in dental augmentation procedures.
Calcium sulfate is the simplest synthetic bone graft material with the longest history of safe use in medicine and dentistry, spanning more than 110 years. It was first used in 1893 by Dreesmann to obliterate bone cavities caused by tuberculosis.18,19 Calcium sulfate exists in three different phases: anhydride, dihydrate, and hemihydrate. Medical-grade calcium sulfate is highly biocompatible, bioresorbable, and osteoconductive. It has a good reputation in bone regenerative techniques because of its safety, moldability, and its complete resorption followed by newly formed bone.20,21 In experiments, calcium sulfate has also been shown to stimulate bone growth when placed in contact with bone or periosteum.22 At 12 months, bone defects filled with calcium sulfate had 99% of the graft resorbed and replaced by 88% bone23 after surgical orthopedic treatment. The resorption profile of calcium sulfate matches the rate at which the host environment24 can lay down bone around the implant.25,26 For example, in dogs, complete resorption is achieved within 4 months.18 Ricci et al27 reported formation of a mineralized hyaluronic acid (HA)-like latticework as the calcium sulfate dissolved.
In the oral cavity, other bone replacement grafts have been used for a multitude of purposes with varying results. Many have been shown to preserve alveolar volume after tooth extraction.7,28 However, there are tremendous differences in how they react biologically in the body. One paper has shown more vital bone formation apically than coronally in the ABBM-grafted socket.14 Histologic evaluation also revealed the nonresorption of anorganic bovine bone mineral at the 9-month evaluation time point. These studies all concur that complete socket preservation was not obtained with the studied techniques and materials.
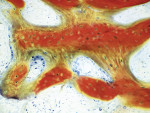
BondBone is a granulated powder functioning as a scaffold for bone regeneration in dental procedures. It is self-reinforced, and, therefore, both sets and remains hard and intact in the presence of blood and saliva. This enables it to preserve the desired 3-D space throughout the healing period. In many cases, it does not require membrane coverage, therefore reducing working time and costs. The resulting unique porous structure and chemical composition determine the strength and bioresorption period that beneficially influences the bone regeneration rate. The composition BondBone, characterized by a controlled, predetermined setting time, strength, and resorption rate, can be used beneficially in a variety of cases during repair of bone defects. Figure 3 presents scanning electromicrographs of a cured sample at 20-micron magnification. An overall rough and uneven microstructure of the bulk structure is shown, exhibiting large amorphous pores (ranging from 300 μm to 800 μm in diameter). The needle-shaped, strongly intergrown crystals of calcium sulfate dihydrate determine the micro-pores of various sizes (from 1 μm to 50 μm) and shapes. The calculated average volumetric porosity was 46% ± 1%.
Patients must make informed decisions on the materials that surgeons place with respect to the origin of these products and their expected biologic results. In the cases shown, vital bone was formed in all re-entered regenerated sites. In the maxillary molar site, 32% vital bone was formed and only 8% residual graft remained. In the mandibular molar site, 51% vital bone resulted, with less than 1% remaining bone replacement graft material; this contrasts to studies with bovine graft materials, where 25% to 35% residual graft has been shown at all time periods.3 Additional studies are necessary to compare vital bone formation in sockets grafted with this calcium sulfate mixture to other graft materials and the long-term dimensional stability of the bone regenerated. Within the limits of this study, it can be concluded that BondBone is a viable material for socket augmentation.
Conclusions
These techniques of extraction and simultaneous graft and barrier placement are very predictable for restoring the volume of the alveolar ridge. Some of the patients in this series were left with calcium sulfate exposed to the oral cavity. The critical sized defect is not known for this material, as it partially depends on the protection of any prosthetic devices over the graft. If it is not desired that primary closure be obtained or it cannot be maintained—leaving a large area of calcium sulfate exposed—the surgeon may benefit by placement of a dense PTFE barrier over the grafted site,10,11 which can be safely left partially exposed to the oral environment. The presented cases demonstrate the clinical and biologic advantages of this novel calcium sulfate graft material.
Clinically, the sockets maintained volume and all had similar density when drilled for the placement of dental implants. In this 4-month case study, the predictable formation of vital bone in treated extraction sockets was only shown in a small sample of cases treated. As the histologic results are validated with radiographic interpretation of other cases, the biphasic calcium sulfate graft material fully resorbs and is replaced by vital bone in the 4-month time period used. The biologic processes that leave the sites filled with vital bone in this timeframe have led to a 100% success rate in implant placement and loading. Additionally, this bone has maintained its integrity radiographically and enabled support of keratinized tissue with no dimensional alterations over the studied time period and well beyond (Figure 18). BondBone is simple and effective to use in treating extraction defects before dental implant placement, and is a viable material for socket augmentation.
Acknowledgements
The authors wish to thank mentors and colleagues, who continue to stimulate the quest for scientific knowledge in regenerative procedures. They include Drs. S. Froum, D. Callan, J. Ricci, J. Sottosanti, D. Anson, A. Piattelli, D. Tarnow, D. Galasso, P. Coelho, and countless other dentists and those behind the scenes who have assisted in histologic processing and analysis of other specimens, including G. Turner.
Disclosure
Several of the authors have received honoraria from MIS Implant Technologies, Osteogenics, and/or Biomet 3i.
References
1. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7):990-999.
2. Araújo M, Linder E, Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2009;20(1):1-6.
3. Artzi Z, Givol N, Rohrer MD, et al. Qualitative and quantitative expression of bovine bone mineral in experimental bone defects. Part 2: Morphometric analysis. J Periodontol. 2003;74(8):1153-1160.
4. Babbush CA. Histologic evaluation of human biopsies after dental augmentation with a demineralized bone matrix putty. Implant Dent. 2003;12(4):325-332.
5. Piattelli A, Scarano A, Piattelli M. Microscopic and histochemical evaluation of demineralized freeze-dried bone allograft in association with implant placement: a case report. Int J Periodontics Restorative Dent. 1998;18(4):355-561.
6. Horowitz RA, Mazor Z, Miller RJ, et al. Clinical evaluation alveolar ridge preservation with a beta-tricalcium phosphate socket graft . Compend Contin Educ Dent. 2009;30(9):588-594.
7. Vance GS, Greenwell H, Miller RL, et al. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2004;19(4):491-497.
8. Anson D. Using calcium sulfate in guided tissue regeneration:a recipe for success. Compend Contin Educ Dent. 2000;21(5):365-376.
9. Sottosanti JS. Aesthetic extractions with calcium sulfate and the principles of guided tissue regeneration. Pract Periodontics Aesthetic Dent. 1993;5(5):61-69.
10. Horowitz RA. Extraction environment enhancement: critical evaluation of early socket healing in long-term barrier-protected extraction sockets. Compend Contin Educ Dent. 2005;26(10):703-713.
11. Bartee BK. The use of high-density polytetrafluorethylene membrane to treat osseous defects: clinical reports . Implant Dent. 1995;4(1):21-26.
12. Hoffmann O, Bartee BK, Beaumont C, et al. Alveolar bone preservation in extraction sockets using non-resorbable dPTFE membranes: a retrospective non-randomized study. J Periodontol. 2008;79(8):1355-1369.
13. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000;71(6):1015-1023.
14. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets: Part 2. Histochemical observations at 9 months. J Periodontol. 2001;72(2):152-159.
15. Artzi Z, Givol N, Rohrer MD, et al. Qualitative and quantitative expression of bovine bone mineral in experimental bone defects. Part 1: Description of a dog model and histological observations. J Periodontol. 2003;74(8):1143-1152.
16. Rohrer MD, Schubert CC. The cutting-grinding technique for histological preparation of undecalcified bone and bone-anchored implants: improvement in instrumentation and procedures. Oral Surg Oral Med Oral Pathol. 1992;74(1):73-78.
17. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11(4):318-326.
18. Peltier LF. The use of plaster of paris to fill large defects in bone. Am J Surg. 1959;97(3):311-315.
19. Dreesmann H. Ueber Knochenplombierung. Bietr Klin Chir. 1892;9:804-810.
20. Yoshikawa G, Murashima Y, Wadachi R, et al. Guided bone regeneration (GBR) using membranes and calcium sulfate after apicectomy: a comparative histomorphometrical study. Int Endod J. 2002;35(3):255-263.
21. Pecora GE, De Leonardis D, Della Rocca C, et al. Short-term healing following the use of calcium sulfate as a grafting material for sinus augmentation: a clinical report. Int J Oral Maxillofac Implants. 1998;13(6):866-873.
22. Coetzee AS. Regeneration of bone in the presence of calcium sulfate. Arch Otolaryngol. 1980;106(7):405-409.
23. Kelly CM, Wilkins RM, Gitelis S, et al. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001;(382):42-50.
24. Silveira RL, Machado RA, Silveira CR, Oliveira RB. Bone repair process in calvarial defects using bioactive glass and calcium sulfate barrier. Acta Cir Bras. 2008;23(4):322-328.
25. Bahn SL. Plaster: a bone substitute . Oral Surg Oral Med Oral Pathol. 1966;21(5):672-681.
26. Tay BK, Patel VV, Bradford DS. Calcium sulfate- and calcium phosphate-based bone substitutes. Mimicry of the mineral phase of bone. Orthop Clin North Am. 1999;30(4):615-623.
27. Ricci JL, Alexander H, Nadkarni P, et al. Biological mechanisms of calcium sulfate replacement by bone. In: Bone Engineering. Davies JE, ed. Toronto, Canada: Em Squared, Inc; 2000:332-344.
28. Fickl S, Zuhr O, Wachtel H, et al. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J Clin Periodontol. 2008;35(10):906-913.
About the Authors
Robert A. Horowitz, DDS
Departments of Periodontics and Implant Dentistry
Oral Surgery
New York University College of Dentistry
New York, New York
Private Practice
Periodontics and Implant Dentistry
Scarsdale and New York, New York
Michael D. Rohrer, DDS, MS
Professor and Director, Division of Oral and Maxillofacial Pathology, Director
Hard Tissue Research Laboratory
University of Minnesota School of Dentistry
Minneapolis, Minnesota
Hari S. Prasad BS, MDT
Senior Researcher
Department of Hard Tissue Research Laboratory
University
of Minnesota School of Dentistry
Minneapolis, Minnesota
Nick Tovar, PhD
Senior Researcher
Department of Biomaterials and Biomimetics
New York University College of Dentistry
New York, New York
Ziv Mazor, DMD
Private Practice
Periodontics and Implant Dentistry
Ra’anana, Israel