Efficacy of the Use of a Water Flosser in Addition to an Electric Toothbrush on Clinical Signs of Inflammation: 4-Week Randomized Controlled Trial
Deborah M. Lyle, RDH, MS; Jimmy G. Qaqish, BSc; C. Ram Goyal, BDS; and Reinhard Schuller, MSc
Abstarct
Purpose: This study compared the use of an oscillating-rotating powered toothbrush and a water flosser to the use of an oscillating-rotating powered toothbrush on the reduction of clinical signs of inflammation and plaque.Methods: Seventy adult participants (N = 70) completed this examiner-blind, two-group, parallel clinical trial. The participants were randomized into either the water flosser + oscillating-rotating powered toothbrush (WF) group or the oscillating-rotating powered toothbrush only group (OR). Inflammation was measured by bleeding on probing (BOP) and modified gingival index (MGI) at baseline, 2 weeks, and 4 weeks. Plaque was scored using Rustogi Modification of the Navy Plaque Index (RMNPI) at the same timepoints. Data was reported for whole mouth, areas (marginal and proximal), and surfaces (facial and lingual). A post-study Likert scale questionnaire was completed at the 4-week visit.Results: Both groups demonstrated significant reductions in BOP, MGI, and RMNPI from baseline to 4 weeks for whole mouth (P < .001). The WF group was 37% more effective at reducing BOP, 36% for MGI, and 33% for RMNPI than the OR group at 4 weeks for whole mouth (P < .001; except RMNPI P = .003). Additionally, the WF group was significantly more effective at reducing proximal BOP (37%, P < .001), MGI (46%, P < .001), and RMNPI (52%, P = .004) compared to OR at 4 weeks. The questionnaire revealed subjects in both groups felt the device was easy to use, the instructions were clear, and their mouth felt fresh and clean. There were no adverse events reported during the study. Conclusion: An oral hygiene regimen consisting of the use of a water flosser in addition to an oscillating-rotating powered toothbrush significantly improved gingival health. The products used in both groups were effective and well-tolerated by the study population.
Toothbrushing is effective in reducing levels of dental plaque to help maintain oral health.1 Manual toothbrushing can reduce plaque scores by approximately 42% after a single use.2 A meta-analysis has not been published on the reduction of gingival inflammation by manual toothbrushing. Individual studies do provide evidence for the reduction of gingival inflammation when judicious brushing technique is used.1
Power toothbrushing reduces plaque scores by 46% on average following a single brushing use. It has been reported that power toothbrushes have a slight advantage over manual toothbrushes for reducing plaque and gingivitis.2 In short-term studies of 1 to 3 months power toothbrushes had significantly greater reduction in plaque of 11% and gingivitis of 6% compared to manual toothbrushes.1,2 Similarly, in studies ≥3 months in duration the difference for plaque was 21% and gingival inflammation was 11%.
Toothbrushing is limited by the inability of the brush to reach subgingivally and interdentally. Traditionally, use of manual brushing and dental floss has been the standard of care. However, recent systematic reviews have shown that this is not supported by the evidence.3,4
Like toothbrushes, interdental aids have been reviewed in randomized clinical trials, systematic reviews, and meta-analyses. A 2008 systematic review found the addition of dental flossing to toothbrushing compared to toothbrushing alone did not show an additional benefit for either plaque or clinical measurements of gingival inflammation.3 A subsequent systematic review found some evidence that there was a reduction in gingival inflammation for toothbrushing and flossing compared to toothbrushing alone, but it was based on poor-quality studies and unreliable evidence.4
There is some evidence from low-quality studies that supplementing toothbrushing with the use of interdental brushes (IDBs) is more effective in reducing plaque scores and gingival bleeding compared to manual toothbrushing alone.5 In a systematic review the addition of water flossing to manual brushing showed no effect on visible plaque, but a positive trend was seen in improving gingival health versus brushing alone.6 This review included water flossers or oral irrigators with different modes of action. Subsequent randomized controlled trials have compared the addition of a water flosser to a manual or powered toothbrush and showed significantly better reductions in plaque, gingival bleeding, and inflammation compared to manual or power brushing alone.7,8
A meta-review addressed the effect of mechanical interdental plaque removal in addition to toothbrushing on managing gingivitis. Based on the available systematic reviews the IDB was the most effective method reported.9 However, a network meta-analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation found IDB and water flosser ranked high and wood sticks and floss ranked near zero for reducing gingival bleeding.10
A water flosser has been compared to dental floss,11-13 interdental brushes,14-16 and airfloss17-19 and consistently demonstrated superiority in reduction of plaque and gingival inflammation scores. It has also been evaluated as an adjunct to manual toothbrushing and sonic toothbrushing.7,8 The present study was designed to evaluate the effectiveness of the use of a water flosser in addition to an oscillating-rotating toothbrush on clinical parameters of inflammation.
Methods and Materials
Ethical Aspects
This study was conducted approximating Good Clinical Practice Guidelines and the ethical principles of the Declaration of Helsinki (1965; Helsinki, Finland) as well as the recently revised version (2013; Fortaleza, Brazil), and applicable local regulations of Health Canada. The study was approved by All Sum Research Center Institutional Review Board (IRB) and registered at www.clinicaltrials.gov (NCT040032989).
The study was conducted from June-July 2019 at All Sum Research Center, Mississauga, Ontario, Canada. Participants were recruited from the center's database of residents in and around Mississauga who met the inclusion criteria. All participants read and signed an informed consent form, a copy of which was given to each participant. Participants were assigned a unique study number to ensure anonymity. The sponsor representative monitored the study.
Study Design
This was an examiner-blind, single-center, two-group, parallel, 4-week randomized controlled clinical trial. Allocation was based on a randomization scheme and no stratification was applied. The examiner was blinded to the treatment allocation and records of previous examinations were kept separate from the current forms. Participants were told and reminded at each visit not to disclose or discuss the product assignment with the examiner. The statistician was off site and received the case report forms at the end of each visit.
Medical history and an intra- and extraoral examination were conducted at the screening appointment. No radiographic data was used for inclusion or exclusion in this study. If the subject qualified, agreed to participate, and signed the consent form he or she was immediately enrolled in the study, and baseline data was collected. Participants were provided their assigned products and used them for the first time. Each participant was scheduled for the 2-week and 4-week visits (+/- 1 day).
Any adverse events were reported, documented, and/or followed-up according to US Food and Drug Administration (FDA) Guidance for Clinical Investigators, Sponsors, and IRBs.20 Study personnel confirmed participants prior to their appointment by a phone call and reminded them of their appointment date and time, to refrain from using the oral hygiene devices for 12 to 14 hours prior to the appointment, and to bring all study material to the visit.
Sample Size and Statistical Analysis
A total of 71 patients were assessed for eligibility. One was excluded for not meeting inclusion criteria. Participants were randomized using a simple computer-generated randomization schedule by the statistician. Based on the randomization schedule, 70 patients were randomly assigned in a 1:1 ratio. With 35 subjects per group, the study had 90% power to detect an average reduction in the percent of sites with bleeding on probing (BOP) of 25%.
The initial comparison was the mean change between the two groups, utilizing one-way analysis of variance (ANOVA). The arcsine transformation was used to stabilize the variances of the percentage data. The transformed data was used in the analysis; however, tables show the mean of subject-specific percentages for the groups. P values of P < .05 were considered statistically significant.
Study Population
Systemically healthy nonsmoking male and female adults between the ages of 18 and 70 were enrolled if they met the eligibility criteria and were able to provide written informed consent prior to participation. Inclusion criteria included a minimum of 50% BOP, 1.75 modified gingival index (MGI), and 0.60 Rustogi Modification of the Navy Plaque Index (RMNPI), as well as at least 20 scoreable teeth (not including third molars) with probing depth ≤4 mm and no partial dentures or orthodontic fixed or removable appliances. Participants agreed to delay dental prophylaxis, elective dental treatment, and cosmetic procedures (ie, tooth whitening). They also agreed to refrain from using any non-study dental device or oral care products, return for scheduled visits, and comply with study procedures.
Participants with a systemic disease (ie, diabetes, autoimmune disease, cardiomyopathy), pregnant or breastfeeding at time of the study, or taking medications that could influence gingival health were not enrolled in the study. Those with a history of antibiotic use within 6 months of the start of the study were excluded. Patients with obvious advanced periodontitis or carious lesions were referred to their dentist of record and not enrolled in the study.
Clinical Assessment
At each of the three visits the participants were evaluated for gingival inflammation and plaque accumulation. Gingival inflammation was evaluated by BOP and MGI. BOP was measured using a UNC probe and recorded as either positive or negative (0 = no bleeding, 1 = bleeding). Recordings were taken at six sites per tooth (mesial-facial, mid-facial, distal-facial, mesial-lingual, mid-lingual, and distal-lingual). Bleeding was recorded within 30 seconds of probing. The percentage of bleeding was determined by dividing the number of bleeding sites by the total number of probable sites.
MGI was scored using a 0-4 scale with: 0 = absence of inflammation; 1 = mild inflammation, ie, slight change in color, little change in texture of any portion of but not the entire marginal or papillary gingival unit; 2 = mild inflammation, ie, criteria as for 1 but involving the entire marginal or papillary unit; 3 = moderate inflammation, ie, glazing, redness, edema, and/or hypertrophy of the marginal or papillary gingival unit; and 4 = severe inflammation, ie, marked redness, edema, and/or hypertrophy of the marginal or papillary gingival unit, spontaneous bleeding, congestion, or ulceration.
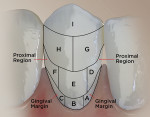
The RMNPI measures nine segments per surface (facial and lingual). This provided data for the whole tooth, proximal region, and gingival margin (Figure 1).
Study Products
Participants in the WF group were provided a water flosser (Waterpik® Aquarius Water Flosser, Water Pik, Inc., waterpik.com) and an oscillating-rotating electric toothbrush (Oral-B® Pro 2000 with the Precision Clean brush head, Procter & Gamble, oralb.com). Participants in the OR group were provided the oscillating-rotating electric toothbrush only. The water flosser is a power-driven device that produces a pulsating stream of water under pressure that is directed at the gingival margin to clean interdentally and subgingivally. It generates a compression and decompression phase that allows for expedient removal of plaque and debris.
The oscillating-rotating electric toothbrush is designed to clean each tooth individually moving from one tooth to another in a systematic fashion. It features a 2-minute timer and a quadrant indicator to support whole-mouth cleaning. The devices chosen for this study are popular with consumers and readily commercially available. At the time of the study, retail market data showed the Precision Clean brush head was the most popular replacement brush head in the Oral-B line. Both products have the American Dental Association (ADA) Seal of Acceptance.
After being provided the product and instruction booklets the participants were told to read the instructions and use the product. The toothbrush was used on the daily clean setting and the water flosser was used on setting 8. The participants were supervised to ensure they read the instructions and used the product but received no further directions. This was designed to simulate real-world purchase and home use of the products. All participants were provided an ADA standard toothpaste (Colgate® Cavity Protection, Colgate-Palmolive, colgate.com).
Results
All the participants completed the study (N = 70). None were lost to follow-up or discontinued intervention, nor were any excluded from analysis. There were no differences between groups at baseline for clinical parameters or participant demographics (Table 1). No adverse events were reported by the participants or found by the examiner during the study.
Bleeding on Probing
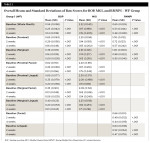
Both groups had a statistically significant reduction in BOP within group at 2 weeks and 4 weeks (Table 2 and Table 3). The reductions for the OR group ranged from 50.7% to 59.8% and the WF group from 68.8% to 83.6% at 4 weeks. The WF group was significantly more effective than the OR group for all areas measured except lingual marginal at 4 weeks (Table 4).
Gingival Index
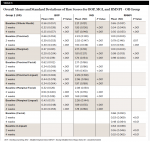
Both groups had a statistically significant reduction in MGI within group at 2 weeks and 4 weeks. The reductions for the OR group ranged from 11.7% to 21% and the WF group from 16.8% to 26.2% at 4 weeks. The WF group was significantly more effective than the OR group for all areas measured except lingual proximal and lingual marginal at 4 weeks (Table 5).
Plaque Index
Both groups had a statistically significant reduction in RMNPI within group at 2 weeks and 4 weeks. The reductions for the OR group ranged from 2.2% to 28.2% and the WF group from 5% to 42.8% at 4 weeks. The WF group was significantly more effective than the OR group for all areas measured at 4 weeks (Table 6).
Post-Study Questionnaire
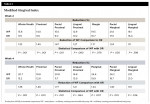
The post-study Likert scale questionnaire revealed that most participants felt both products were easy to use and made their mouth feel fresh and clean. There were no significant differences between the groups for the first, second, third, and fifth questions. There was, however, a significant difference for the fourth question where 91% of participants in the WF group would continue to use the products after the study, but only 60% of the OR group would continue using the product (P = .003) (Table 7).
Discussion
The primary outcome of this parallel, 4-week randomized controlled clinical trial was the effectiveness of an oral hygiene regimen that added the use of a water flosser to an oscillating-rotating powered toothbrush on the level of naturally occurring mild to moderate gingivitis versus use of an oscillating-rotating powered toothbrush only. Both oral hygiene regimens showed a significant reduction in gingival bleeding and inflammation, and there were no reports of adverse events related to the study products or otherwise.
Based on the results of this study it is evident that the oscillating-rotating powered toothbrush is significantly effective in reducing gingivitis as measured by BOP and MGI, is easy to use, and provides a clean, fresh feeling for users. The differences between the groups showed that the use of an oscillating-rotating powered toothbrush is significantly enhanced by the additional use of a water flosser to clean inaccessible areas interdentally and subgingivally.
These results are in line with other studies that measured the use of a water flosser as a supplement to toothbrushing. Use of a water flosser and a manual toothbrush were 3.13 times as effective for BOP, 2.69 times for MGI, and 2.44 times for RMNPI compared to manual brushing alone.8 Use of a sonic toothbrush and water flosser demonstrated 34% and 70% better reduction in BOP, 23% and 48% for MGI, and 18% and 52% for RMNPI compared to use of a sonic toothbrush alone. The different results for each index is related to the two brands of sonic toothbrushes tested.7
Use of a water flosser has also been compared to toothbrushing and flossing, toothbrushing and interdental brushing, and toothbrushing and airflossing. The use of manual toothbrushing and a water flosser consistently demonstrate significantly better results than manual toothbrushing and flossing,11-13 even for people with orthodontic fixed appliances and implants.21,22 A newly designed oral hygiene device that has a water flosser jet tip embedded in the sonic brush head has been shown to be significantly more effective than sonic brushing alone (38% for BOP and MGI, 36% for RMNPI) or manual brushing and flossing (107% for BOP, 109% for MGI, 116% for RMNPI) over 4 weeks.23
Three studies have compared manual toothbrushing with use of a water flosser and manual toothbrushing with airflossing.17-19 Both combinations were effective in reducing clinical parameters of gingival inflammation within group over 4 weeks. The water flosser and manual toothbrush were significantly more effective than the manual toothbrush and airfloss for removing plaque after a single use and reducing plaque, BOP, and MGI over 4 weeks.
In the present study the groups' answers were comparable for four of the five questions on the post-study questionnaire. There was a difference in the groups on whether they would continue to use the product(s) after the study, with most of the WF group providing a positive response compared to 60% in the OR group. The reason for this is not evident as there were no open-ended questions and 40% (n = 14) responding neither agreed nor disagreed for not continuing use.
Limitations
The participants could not be blinded to the devices but did use the same oscillating-rotating powered toothbrush in both groups. The study duration was 4 weeks, which is sufficient to identify changes in gingival health. This is the time required by the ADA seal of acceptance guidelines for powered interdental devices to demonstrate efficacy.24 Minimal gingival assessment outcomes for efficacy are 10% reduction using the MGI and 15% reduction using the Löe and Silness Gingival Index25 compared to the control group. This study surpassed the minimum with a MGI score of 36%.
A longer-term study may answer the question of whether the level of improvement continues and eventually reaches a state of periodontal and gingival health (<10% BOP and an intact periodontium) as outlined in the 2017 World Workshop new classification scheme for periodontal and peri-implant diseases and conditions.26 A longer prospective study may also address the issue of adherence and motivation to an oral hygiene regimen.
Conclusion
This study adds to the body of evidence that brushing with either a manual or powered toothbrush does not adequately maintain oral health. It demonstrated that an oscillating-rotating powered toothbrush is very effective and the addition of water flossing to brushing with an oscillating-rotating powered toothbrush has a statistically positive effect on oral health status for people with mild to moderate gingivitis.
Disclosure
The authors report no conflicts of interest. The study was performed by All Sum Research Center Ltd. through a research grant from Water Pik, Inc., which provided the study products. Deborah M. Lyle is an employee of Water Pik, Inc.
About the Authors
Deborah M. Lyle, RDH, MS
Director of Professional and Clinical Affairs, Water Pik, Inc., Fort Collins, Colorado
Jimmy G. Qaqish, BSc
Vice President of Operations, All Sum Research Center Ltd., Mississauga, Ontario, Canada
C. Ram Goyal, BDS
President and Examiner, All Sum Research Center Ltd., Mississauga, Ontario, Canada
Reinhard Schuller, MSc
Owner and Statistician, Reinhard Schuller Consulting, Toronto, Ontario, Canada
References
1. Van der Weijden FA, Slot DE. Efficacy of homecare regimens for mechanical plaque removal in managing gingivitis a meta review. J Clin Periodontol. 2015;42(suppl 16):S77-S91.
2. Chapple IL, Van der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42(suppl 16):S71-S76.
3. Berchier CE, Slot DE, Haps S, Van der Weijden GA. The efficacy of dental floss in addition to a toothbrush on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6(4):265-279.
4. Sambunjak D, Nickerson JW, Poklepovic T, et al. Flossing for the management of periodontal diseases and dental caries in adults. Cochrane Database Syst Rev. 2011;(12):CD008829.
5. Popklepovic T, Worthington HV, Johnson TM, et al. Interdental brushing for the prevention and control of periodontal diseases and dental caries in adults. Cochrane Database Syst Rev. 2013;(12):CD009857.
6. Husseini A, Slot DE, Van der Weijden GA. The efficacy of oral irrigation in addition to a toothbrush on plaque and the clinical parameters of periodontal inflammation: a systematic review. Int J Dent Hyg. 2008;6(4):304-314.
7. Goyal CR, Lyle DM, Qaqish JG, Schuller R. The addition of a water flosser to power tooth brushing: effect on bleeding, gingivitis, and plaque. J Clin Dent. 2012;23(2):57-63.
8. Goyal CR, Qaqish JG, Schuller R, Lyle DM. Evaluation of the addition of a water flosser to manual brushing on gingival health. J Clin Dent. 2018;29(4):81-86.
9. Salzer S, Slot DE, Van der Weijden FA, Dorfer CE. Efficacy of inter-dental mechanical plaque control in managing gingivitis-a meta-review. J Clin Periodontol. 2015:42(suppl 16):S92-S105.
10. Kotsakis GA, Lian Q, Ioannou AL, et al. A network meta-analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation. J Periodontol. 2018;89(5):558-570.
11. Barnes CM, Russell CM, Reinhardt RA, et al. Comparison of irrigation to floss as an adjunct to tooth brushing: effect on bleeding, gingivitis, and supragingival plaque. J Clin Dent. 2005;16(3):71-77.
12. Rosema NA, Hennequin-Hoenderdos NL, Berchier CE, et al. The effect of different interdental cleaning devices on gingival bleeding. J Int Acad Periodontol. 2011;13(1):2-10.
13. Goyal CR, Lyle DM, Qaqish JG, Schuller R. Evaluation of the plaque removal efficacy of a water flosser compared to string floss in adults after a single use. J Clin Dent. 2013;24(2):37-42.
14. Goyal CR, Lyle DM, Qaqish JG, Schuller R. Comparison of water flosser and interdental brush on reduction of gingival bleeding and plaque: a randomized controlled pilot study. J Clin Dent. 2016;27(2):61-65.
15. Slot DE, Lyle DM, Van der Sluijs E, et al. Water flosser compared to interdental brush on bleeding scores and gingival abrasion [abstract]. J Dent Res. 2018;97(spec iss B):Abstract 0622.
16. Lyle DM, Goyal CR, Qaqish JG, Schuller R. Comparison of water flosser and interdental brush on plaque removal: a single-use pilot study. J Clin Dent. 2016;27(1):23-26.
17. Goyal CR, Lyle DM, Qaqish JG, Schuller R. Efficacy of two interdental cleaning devices on clinical signs of inflammation: a four-week randomized controlled trial. J Clin Dent. 2015;26(2):55-60.
18. Goyal CR, Qaqish J, Schuller R, Lyle DM. A direct comparison of two interdental cleaning devices on clinical signs of inflammation: a four-week randomized controlled trial. Ann Clin J Dent Health. 2018;(7):10-15.
19. Sharma NC, Lyle DM, Qaqish JG, Schuller R. Comparison of two power interdental cleaning devices on the reduction of gingivitis. J Clin Dent. 2012;23(1):22-26.
20. Guidance for Clinical Investigators, Sponsors, and IRBs. Adverse Event Reporting to IRBs-Improving Human Subject Protection. US Dept of Health and Human Services. Food and Drug Administration. January 2009. https://www.fda.gov/media/72267/download. Accessed November 21, 2019.
21. Sharma NC, Lyle DM, Qaqish JG, et al. Effect of a dental water jet with orthodontic tip on plaque and bleeding in adolescent patients with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2008;133(4):565-571.
22. Magnuson B, Harsono M, Stark PC, et al. Comparison of the effect of two interdental cleaning devices around implants on the reduction of bleeding: a 30-day randomized clinical trial. Compend Contin Educ Dent. 2013;34(spec iss 8):2-7.
23. Goyal CR, Qaqish JG, Schuller R, Lyle DM. Comparison of a novel sonic toothbrush with a traditional sonic toothbrush and manual brushing and flossing on plaque, gingival bleeding, and inflammation: a randomized controlled clinical trial. Compend Contin Educ Dent. 2018;39(suppl 2):14-22.
24. American Dental Association Council on Scientific Affairs. Acceptance Program Requirements: Powered interdental cleaners or oral irrigators. July 2019. https://www.ada.org/en/science-research/ada-seal-of-acceptance/how-to-earn-the-ada-seal/guidelines-for-product-acceptance. Accessed December 4, 2019.
25. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Ondontol Scand. 1963;21:533-551.
26. Trombelli L, Farina R, Silva CO, Tatakis DN. Plaque-induced gingivitis: case definition and diagnostic considerations. J Clin Periodontol. 2018;45(suppl 20):S44-S67.