A Report of Five Cases from an Ongoing Prospective Clinical Study on a Novel Pink Biomimetic Implant System
Mariano A. Polack, DDS, MS | E. Todd Scheyer, DDS, MS | Kevin G. Murphy, DDS, MS | Joseph M. Arzadon, MD, DDS | Alan L. Rosenfeld, DDS | George A. Mandelaris, DDS, MS
SUPPORTED BY AN EDUCATIONAL GRANT FROM KEYSTONE DENTAL, INC.
ABSTRACT:
Color discrepancies between peri-implant soft tissues and materials used in implants, abutments, and restorations may influence overall esthetics at the implant–soft-tissue interface, particularly in the esthetic zone. In an ongoing 5-year multicenter prospective post-marketing surveillance study of 120 adult male and female participants at eight sites in the United States (total of 168 implants placed), the authors have been evaluating anterior and posterior single-tooth implants using a novel pink osteoconductive implant system (in clinical use since 2010) that features a variety of pink components, developed with the objective of improving peri-implant soft-tissue esthetics. Clinical analyses of the 18-month interim survival rates, marginal bone and soft-tissue level changes, and esthetics have been completed, showing an overall success rate among all of the implanted sites of 95.8%. This case series aims to summarize data on implant survival, probing-derived and radiographically assessed marginal bone and soft-tissue level changes, and qualitative photographic evidence of post-restorative soft-tissue esthetic outcomes by presenting a snapshot of five representative cases (two anterior and three posterior), at 18 months from the start of this study. Four of the five cases described here involve teeth visible in full smile and comprise three maxillary incisors and two maxillary premolars. The remaining case was a relatively straightforward mandibular first-molar replacement. However, all scenarios posed unique esthetic challenges. Three subjects received immediate implants; the remaining two required post-extraction regenerative procedures. Gingival inflammation, bleeding on probing, and plaque were infrequently observed throughout the treatment period. Implant success and stability, alveolar bone-level stability, soft-tissue height and attached-gingiva width stability, and peri-implant soft-tissue esthetic outcomes were uniformly excellent at the 18-month follow-up visit. Data from the entire ongoing multicenter study population will be published both at 3 years and at study completion at 5 years. Those results will be necessary to assess any statistical differences in tissue changes and/or bone levels and apply meaningful interpretation to aggregate observed qualitative colorimetric soft-tissue parameters associated with this implant system.
Introduction
Despite the high predictability of tooth replacement with osseointegrated implants,1-5 management of tissue esthetics at the facial restoration margin can pose significant challenges for the prosthodontist, restorative dentist, periodontist, and oral and maxillofacial surgeon, and is of particular concern in the esthetic zone. In general, the closer natural shades of hard and soft tissue can be mimicked, the better the esthetic result. Gingival esthetic challenges have been addressed specifically using externally placed pink porcelain on prosthetic components to simulate natural gingiva, with varying degrees of success.6-8 The current system (Genesis®, Keystone Dental, Inc, www.keystonedental.com) addresses a similar goal by modifying internal esthetics within the implant/abutment–free gingival interface.
The proximity of the facial implant–soft-tissue interface to that of a crown margin places an intense focus on harmonization of compatibilities among the inherent colorations of various metals, ceramics, and gingiva in a variety of soft-tissue scenarios. The ideal treatment objective is to make this convergence visually indistinguishable.
Esthetic impact of implant–abutment interface design has been reported in a recently published case series by McGuire et al9; specifically, adherence to a standardized treatment protocol yielded good esthetics with the three different interface designs tested (conical, flat, or platform-switched). One-year results from the larger 5-year randomized clinical trial by Cooper et al10 represented by those cases demonstrated that difference in interface design had significant impact on marginal bone stability but not on gingival mucosal architecture or position (including the apical-most aspect of the facial gingival margin contour, ie, zenith).10
The case series presented here represents another ongoing 5-year clinical study comprising 120 patients who required replacement of one or more anterior or posterior teeth, now approaching completion of 4 years of follow-up to evaluate clinical implant efficacy and soft-tissue esthetics of this unique implant system developed with the objective of overcoming color discrepancy-driven challenges. Three additional representative case reports from this study have been published.11
This system uses a biomimetic implant–bone interface produced by anodic spark deposition or discharge (ASD; also known as microarc oxidation or glow discharge deposition) to the threaded titanium implant surface (BioSpark™, Keystone Dental, Inc)12-16 via electrochemical anodization to form a nanorough, osteoconductive titanium-oxide implant surface rich in calcium and phosphorus ions as a bone interface.13,15,16 In global use since November 2010, this system also features a variety of prefabricated and customizable pink abutments and other restorative components, including implant collars and matching prefabricated customizable titanium abutments. Unless otherwise customized, the transmucosal portion of the abutment and/or the implant collar are uniformly pink throughout the system.
The pink color is produced on the implant surface by a proprietary electrochemical anodization process (AnaTite™, Keystone Dental, Inc), which produces a layer of titanium oxide on the implant surface. The resulting pink coloration also helps mask the gray hue that could be observed with conventional implants under the gingiva of thin-biotype patients, thus offering the clinician an alternative to zirconia for creating, enhancing, and refining gingival esthetics.
Published preclinical studies have evaluated this implant system’s surface in regard to bone-to-implant contact.12,17,18 In vitro studies on cell behavior13,14 and studies on the effects of pink on gingival esthetics have evaluated this system from clinical19,20 and animal-tissue perspectives.21
Spectrophotometric analyses published by Park et al confirmed that there is a measurable difference between the colors of natural maxillary labial gingiva and the surfaces of conventional titanium implants.19 More specifically, colorimetric data reported by Ishikawa-Nagai et al suggest that (in comparison to other colors) light pink coloration of the implant neck produces an optimal color that is clinically indistinguishable from that of natural gingiva.20 Patient-specific shading of the implant collar using a similar approach has also been described in a three-case series published by Sumi et al, who reported such specificity to provide stable gingival esthetics at a 1.5-year follow-up, especially in patients with a thin gingival biotype.22
A case report by Polack published in 2012 specifically evaluated the pink nanorough implant system presented in the current case series (Genesis). An excellent result was achieved in an esthetically demanding case that required multiple extractions and site development for the replacement of four maxillary incisors (using narrow-diameter, 3.8-mm x 13-mm fixtures to replace two laterals, creating a four-unit implant bridge) in a severely resorbed ridge.23
Functional Considerations of Immediate Implant Placement
In the authors’ experience, the aggressive thread pitch of the implant fixture used in this case series also facilitates its efficacy in immediate placement and loading scenarios. Of note, all implants in this multicenter study population have surpassed 3 years of survival, function, and success; the vast majority of them were immediate placements (78%; 22% were staged).
Results of a meta-analysis published by Kinaia et al in 2014 comprising 16 controlled studies suggests that immediate implant placement preserves crestal bone significantly more effectively than implant placement in healed bone after at least 12 months of functional loading.24 Furthermore, this meta-analysis also identified a significant advantage for the use of platform switching in such immediate placement scenarios.24
Preliminary results from an ongoing randomized clinical study by Huynh-Ba et al showed no short-term differences in esthetic outcomes in immediate versus early implant placements.25 Cosyn et al also reported minimal midfacial recession (in two of 25 patients after 3-years’ follow-up) following an immediate implant placement protocol in the anterior maxilla in patients with thick gingival biotypes.26 A recent systematic review by Slagter et al that also encompassed immediate provisionalization reported similar findings.27 Another systematic review by Cosyn et al found conflicting evidence regarding contributory factors to midfacial recession after immediate implant placement but suggested this risk is lowest in patients who have a thick biotype and an intact buccal bone wall and receive immediate provisionalization.28
The implant system used in the current multicenter study incorporates a platform-switch ranging between 0.50 mm and 1.38 mm, depending on implant fixture diameter (IFD):
• IFD = Ø3.8 mm: 0.50 mm PS
• IFD = Ø4.5 mm: 0.57 mm PS
• IFD = Ø5.5 mm: 0.70 mm PS
• IFD = Ø6.5 mm: 1.38 mm PS
Platform switching has become a standard feature in implant component design and has expanded the clinician’s control over crestal bone preservation. Numerous studies29-33 and systematic reviews24,34-37 have reported reduced alveolar crestal bone resorption for platform-switched implants compared with platform-matched implants.
Considerable clinical evidence suggests platform switching has a bone-protective effect. Cappiello et al reported a significant preservation effect (vertical bone loss was 0.72 mm less with platform-switched healing abutments versus controls) in a controlled clinical trial of 131 implants (all placed at the crest) in 45 patients.32 Clinical studies by Prosper et al38 and Canullo et al39 have also demonstrated advantages of platform-switched implants over regular implants with respect to crestal bone stability, with a minimum of 24 months’ follow-up. Recent systematic reviews consistently confirm that implants with platform-switched abutments are associated with better crestal bone preservation than implants with platform-matched abutments.35-37
While platform-switched implant configurations also appear to preserve soft tissue and provide increased control over gingival esthetics according to some reports,40,41 several recent studies tend toward reporting similar tissue-esthetics preservation with platform-switched and other abutment–implant interface designs,9,10,42 which suggests that platform switching favors stable tissue dynamics. However, a study by Zuiderveld et al found platform switching to have no effect on midbuccal mucosal (MBM) measurements 1 year after crown placement; rather, the buccopalatal positioning of the implant itself (ie, more toward the buccal) resulted in a more apically positioned MBM.43
Findings of a systematic review by Prasad et al emphasize the importance of considering a synthesis of factors comprising implant design, occlusal forces, and bone and soft-tissue volumes in optimally preserving crestal bone.44 As a further caveat, even the authors of some recent systematic reviews raise notes of caution about remaining unknowns as to functional specifics of platform switching and stress the need for further and more specific data from clinical studies to evaluate them.34,35
Taken together, these findings offer evidence that the functionality of this implant system in various placement protocols may complement bone- and soft-tissue-preserving effects, with immediate placement in combination with platform switching.
Five-Year Prospective Clinical Study
An ongoing 5-year study continues to evaluate the use of this implant system (168 implants placed in 120 partially edentulous patients). Its objectives include assessment of the 5-year survival rate of this implant system, implant success, incidence of excessive bone loss, peri-implant infection and other complications, incidence of adverse device effects, change in marginal bone level, visual soft-tissue esthetic outcomes, and the number and nature of prosthetic revisions.
Alignment, orientation, and magnification of the periapical radiographic images of all subjects’ implants and alveolar bone levels were standardized by rotating and translating each image such that all were uniformly aligned, oriented, and scaled using a semi-automated program (MATLAB®, MathWorks, Inc, www.mathworks.com/products/matlab/). For angles, imaging differences in both elevation (above or below correct plane) and azimuth (mesial–distal) between images in the same series were computed. All of the images in this data set have a percentage error of less than 3.5%. Clinical analyses of the investigator-reported 18-month interim survival rates, marginal bone and soft-tissue level changes, and esthetics estimate an overall success rate among all sites of 95.8%.
Consistent with other implant designs, the few osseointegration failures observed in the study occurred during the healing period following placement or shortly after prosthetic loading. However, unlike other designs, the location (mandible versus maxilla)45-48 and length of the implant46-49 had no apparent effect on the survival rate. After loading, this implant system has demonstrated a survival rate of more than 99%, based on available data from this ongoing study.
This is primarily a clinical implant survival and efficacy study with hard- and soft-tissue metric endpoints. The study protocol defines implant success as peri-implant bone loss ≤3 mm. Its descriptive endpoints require radiographic and photographic documentation only, and the esthetic results are presented as clinical photographs.
Implant Success and Restorative/Esthetic Results: 18 Months
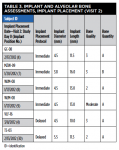
Four female and one male subjects who were either missing or required extraction of one or more natural teeth, who were enrolled in this single-arm multicenter study (22% of all enrolled subjects received immediate implants; the remaining 78% received implants placed with a delayed/staged protocol) according to the inclusion and exclusion criteria listed in Table 1, were selected as representative cases. The implant system evaluated, and both placement approaches, met all criteria for inclusion in the study, as per the study protocol (Table 1).
Baseline characteristics for these five study participants are summarized in Table 2. All subjects were healthy nonsmokers and had unremarkable medical histories except for routine surgeries, treatment of chronic conditions such as hypertension, hypercholesterolemia, and seasonal allergies, and other conditions specified in Table 2.
Extraction indications comprised periodontitis (N = 2) and endodontic pathosis and/or fractured/nonrestorable teeth (N = 4); two patients had combined periodontic–endodontic involvement of the proposed implant sites.
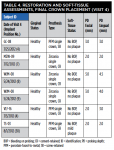
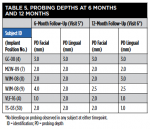
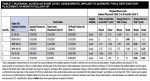
Table 3 and Table 4 summarize implant dimensions, placement protocol, and bone characteristics at Visit 2, and restoration and soft-tissue measurements (including probing depths [PD]) at final crown placement (Visit 4). Table 5 shows PD at 6 and 12 months; Table 6 shows peri-implant PD and other soft-tissue changes at the 18-month assessment (Visit 7). Table 7 summarizes the static marginal bone observations at Visit 7 (ie, positive or negative distance between implant platform shoulder and mesial or distal crestal bone level) compared with dynamic changes that occurred in this distance between implant placement and final restoration placement (Visit 2 to Visit 4) and between final restoration placement and 18-month follow-up (Visit 4 to Visit 7) as calculated by the MATLAB analysis of radiographic assessments at those timepoints. All implants were placed at the crest except for Case 1 (mesial) and Case 2 (distal) (Table 7).
Case Reports
Across all five cases reported here, buccal or labial soft-tissue height was stable or increased by ≥1.23 mm; lingual soft-tissue height was reduced by ≤0.54 mm. Facial and lingual attached-gingiva widths were reduced by ≤1.09 mm and ≤0.52 mm, respectively. Implant-site PDs were ≤4.5 mm at final crown placement and at 6, 12, and 18 months after implant placement. The maximum PD at 18 months was one isolated measurement of 3.5 mm; overall, gingival inflammation, bleeding, and plaque were infrequently observed, and esthetic results were uniformly excellent at the 18-month follow-up visit.
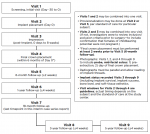
All subjects signed an informed consent document prior to enrollment in the study; that document and the study protocol were approved by an institutional review board at each study center. The study is being conducted in accordance with the United States 21 CFR Parts 11, 50, 56; the Health Insurance Portability & Accountability Act of 1996 (HIPAA); and the Declaration of Helsinki and its amendments, as specified in the most recent meeting of the World Medical Assembly. Figure 1 presents the study flow diagram.
Case 1
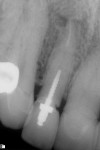
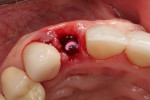
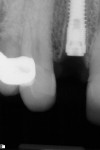
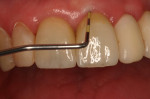
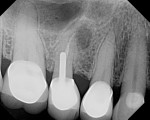
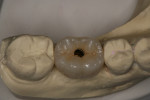
A healthy 69-year-old nonsmoking Caucasian woman presented to one author’s (ALR) periodontal practice with periodontitis involving tooth No. 4. Figure 2 shows a midfacial PD of 3 mm at Visit 1. A PD of 5 mm to 7 mm was present interproximally, with clinical attachment loss of 2 mm to 4 mm; this tooth was also fractured and had a draining sinus tract, and a 7-mm intrabony defect was present on the mesial aspect, suggesting combined periodontic–endodontic involvement (Figure 3). The patient was taking aspirin, trazodone, sertraline, simvastatin, and a calcium supplement (see Table 2 for additional history).
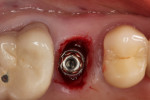
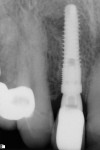
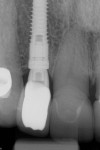
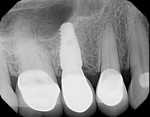
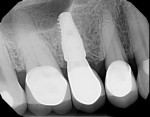
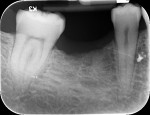
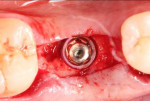
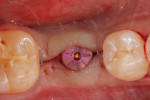
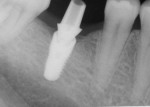
With the patient under local anesthesia and intravenous (IV) sedation, tooth No. 4 was extracted atraumatically, osteotomy was performed for immediate placement of a 4.5-mm x 11.5-mm implant (Genesis) (Figure 4 and Figure 5), followed by bone grafting using a corticocancellous mineralized freeze-dried bone allograft to manage the implant–alveolus discrepancy, and a healing cap was placed. At Visit 3, normal healing and good tissue tone were observed (Figure 6).
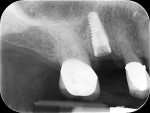
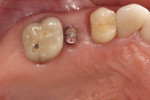
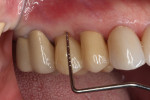
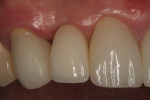
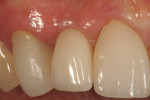
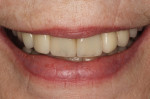
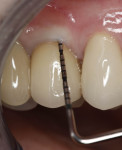
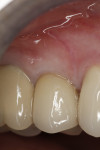
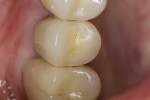
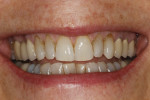
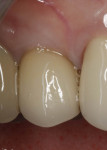
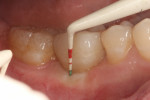
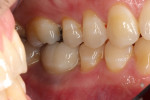
In July 2012 the stock abutment (using the standard platform-switched connection) and final porcelain-fused-to-metal (PFM) crown were placed; gingival health was excellent and showed a midfacial PD of 1 mm (Figure 7). At 18 months (Visit 7) a midfacial probing depth of 3 mm without bleeding was noted (Figure 8). Endodontic treatment was performed on tooth No. 5 after completion of the implant restoration (Figure 9). A subsequent follow-up photograph from January 2015 (Figure 10) shows the facial view of the final crown in occlusion.
Case 2
A healthy 67-year-old nonsmoking Caucasian woman presented to one author’s (KGM) periodontal/prosthodontic practice in January 2012 with a symptomatic maxillary right lateral incisor (tooth No. 7). Her biotype was deemed to be within normal limits, with the attached tissue thickness estimated to be greater than 2 mm, and there was no bleeding on probing (Figure 11).
A periapical radiograph (Figure 12) revealed previous endodontic treatment and periapical pathology. After an endodontic specialist consultation and the poor restorative prognosis being established, the patient elected extraction of this tooth and replacement with an immediate implant. Following an extraction procedure that minimized the trauma to the labial portion of the alveolus of tooth No. 7, an osteotomy was prepared using a SICAT CAD/CAM-generated surgical guide (SICAT/Sirona, www.sicat.com), and a 3.8-mm x 16-mm implant (Genesis) was placed with an insertion torque of 45 Ncm (Figure 13). The osteotomy was performed through the apical area of the alveolus and within the palatal wall. An implant–socket gap approximately 1 mm wide and 5 mm in depth was evident on the distal aspect of the osteotomy immediately after implant placement (Figure 14). No bone graft was used in the osteotomy space, as the labial plate thickness was deemed to be about 2 mm, and the distal socket residual wall had a thick (>2 mm), dense lamina dura; this presentation, in the author’s experience, has a high degree of predictability of bone fill.
Immediate provisionalization was accomplished with a stock Esthetic Contour Ti Abutment (Genesis), which was tried in at the time of implant placement. An egg-shell type provisional was relined over the abutment with a methylmethacrylate self-curing resin. The immediate provisional restoration was inserted and cemented with a temporary cement (Temrex, Temrex Corp, www.temrex.com).
A secondary permanent Esthetic Contour Ti Abutment was modified to receive the all-ceramic restoration (IPS e.max® Ceram, Ivoclar Vivadent, www.ivoclarvivadent.com) layered upon a Procera zirconia coping (NobelProcera, Nobel Biocare, www.nobelbiocare.com). The abutment was 3.3 mm in diameter at the implant–abutment interface, resulting in a 0.5-mm platform switch.
Figure 15 shows the periapical radiograph at final crown placement (Visit 4). The abutment was screwed in place to a torque of 35 Ncm and the cement-retained crown was placed with RelyX™ Unicem (3MESPE, www.3MESPE.com); at this time only mild gingival inflammation was observed (Figure 16). One year later (18-month follow-up), an excellent esthetic result was observed from the facial aspect, with good tissue tone and no gingival recession (Figure 17).
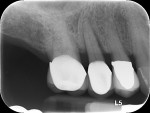
Three years postoperatively, radiographic interpretation suggested maintenance of the crestal bone level and a stable thickness of the labial plate, from the time of implant placement throughout the follow-up period (Figure 18).
Case 3
A healthy 67-year-old nonsmoking Caucasian woman presented to one author’s (MAP) prosthodontic practice in December 2011. Her medical history was significant for several chronic but managed conditions (Table 2); she was taking losartan/hydrochlorothiazide for hypertension, fluoxetine for depression, and nitrofurantoin to treat a bladder infection that was present at the time of her implant surgery.
She had preexisting PFM restorations on teeth Nos. 8 and 9 (Figure 19), both of which had been endodontically treated (Figure 20). In August 2011 the crown on No. 8 dislodged and was recemented on an emergency basis; in November 2011 both crowns (Nos. 8 and 9) dislodged, and both teeth were given a questionable prognosis.
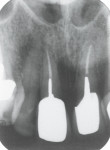
Accordingly, the patient enrolled in the multicenter study in December 2011. She opted for extraction, immediate provisionalization, and immediate loading, and visited both the oral surgeon (JMA) and prosthodontist (MAP) authors’ practices on the same day in January 2012 to consolidate these phases. Teeth Nos. 8 and 9 were extracted under local anesthesia (infiltration with lidocaine 2% with 1:100,000 epinephrine, 3.6 mL). The crowns were removed, then the roots were elevated and extracted. Osteotomy was made in type II (moderate) bone with Class A bone quality; all socket walls were intact. After tapping the sites, two 4.5-mm x 13-mm tapered implants (Genesis) were placed with primary stability of 40 Ncm (Figure 21) and 5-mm healing covers were placed (Figure 22 and Figure 23). The buccal socket gaps were grafted with spongious bone substitute (Bio-Oss®, Geistlich Pharma North America, www.geistlich-na.com). The gingival margins were reapproximated with 4-0 chromic sutures. Postoperative radiographs confirmed proper positioning in the alveolar bone.
Immediately after surgery, the prosthodontist attached prefabricated polymethylmethacrylate (PMMA, tooth-colored) provisional abutments (Temporary Abutment®, Keystone Dental, Inc) with screws. The abutments were hand-tightened and minimally prepared with a diamond bur. Laboratory-fabricated splinted provisional crowns were relined with Jet Acrylic (Lang Dental Manufacturing Co, Inc, www.langdental.com), adjusted, and cemented with eugenol-free zinc-oxide temporary cement (RelyX™ Temp NE, 3M ESPE) on the PMMA temporary abutments. Teflon was used to seal the access holes. The patient was instructed to minimize chewing on these teeth and restrict hard food for 6 weeks.
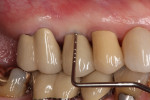
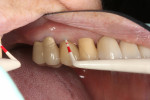
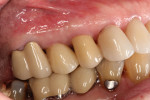
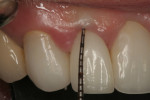
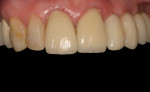
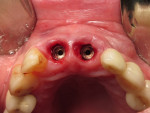
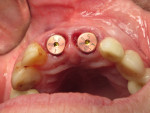
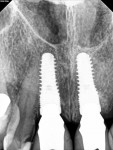
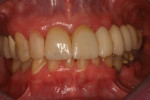
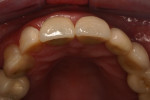
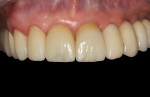
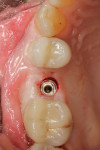
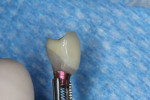
The final impression (closed tray) was obtained in April 2012. The final ceramic crowns (IPS e.max Ceram) and custom porcelain-veneered, regular-diameter (RD) UCLA abutments (Genesis; and Creation CC, Jensen Dental, www.jensendental.com) (Figure 24, shown with PFM crowns and retention screws) were delivered in May 2012. Using a platform-switched connection, the abutments were torqued to 30 Ncm, the access holes sealed with Teflon, and the final crowns cemented with RelyX Unicem. Figure 25 through Figure 28 show the final IPS e.max Ceram crowns from periapical, facial, and incisal views, with a midfacial PD of 3 mm at the 18-month follow-up (Visit 7). A thick biotype is evident in Figure 26, as determined by the inability to detect the outline of the periodontal probe inserted below the restoration’s gingival margin.50 This image also demonstrates an excellent esthetic outcome.
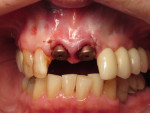
After this visit, additional restorative work was completed on teeth Nos. 6 and 7. Figure 29 and Figure 30 (retracted and smile views, respectively, at 3 years, Visit 8) show the excellent esthetic outcome registered during the 3-year postoperative period.
Case 4
A healthy 65-year-old nonsmoking Caucasian woman was seen by her restorative dentist in December 2011 for a symptomatic maxillary right second premolar (tooth No. 4). The preoperative radiograph showed extensive periapical pathology and resorption (Figure 31).
Her medical history was unremarkable for current conditions; several prior routine surgeries included hysterectomy (Table 2). She was taking raloxifene for osteoporosis prevention and an estradiol hormone-replacement supplement.
In January 2012 tooth No. 4 was extracted. The site was grafted with mineralized freeze-dried cortical bone allograft (OraGraft®, LifeNet Health, www.lifenethealth.org) and covered with resorbable collagen membrane (CollaTape®, Zimmer Biomet, www.zimmerbiomet.com), which was secured with 5-0 plain-gut sutures, followed by flap closure using 4-0 plain-gut sutures.
In February 2012 the patient enrolled in the multicenter study. Using a flapless access approach with the patient under nitrous-oxide sedation, one of the periodontist authors (ETS) removed keratinized tissue from the proposed implant site using a 4-mm biopsy punch. Osteotomy was prepared using a surgical guide for the placement of a 4.5-mm x 10-mm tapered implant (Genesis), which was anchored in the floor of the maxillary sinus (using osteotomes) for optimal primary stability, at an insertion torque of 45 Ncm (Figure 32).
A temporary cylinder abutment was used to fabricate a screw-retained provisional restoration, with the aim of capturing an appropriate esthetic emergence profile. Once this was accomplished, the provisional was secured with a gold screw, which was torqued to 20 Ncm. The occlusion was adjusted to eliminate any centric or excursive contacts.
In June 2012 the provisional was removed, implant insertion torque was verified, and the provisional was reattached. The patient was given the impression parts to bring with her to the fixture-level impression appointment with her restorative dentist.
One month later, the custom UCLA abutment (Figure 33) was attached to the fixture using a platform-switched connection, with an insertion torque of 35 Ncm. The final PFM crown was cemented using methylmethacrylate (C&B-Metabond® Quick! Cement System, Parkell, www.parkell.com). The occlusion and the patient’s nightguard were checked and adjusted to the new restoration.
The midfacial PD at Visit 7 (18-month follow-up) was 2.5 mm, with no bleeding and good gingival tone (Figure 34). Good radiographic osseointegration (Figure 35) and excellent soft-tissue and restoration esthetics were observed at this visit (Figure 36 through Figure 38). The patient was last seen in February 2015 (almost 3 years after implant placement), at which time favorable hard- and soft-tissue levels and optimal esthetics were confirmed clinically and radiographically (Figure 39 and Figure 40).
Case 5
A healthy 43-year-old nonsmoking Caucasian man was seen in the periodontal practice one of the authors (GAM) in February 2012. His medical history was unremarkable except as noted in Table 2; he was taking no medications and had no known drug allergies.
The patient presented with a symptomatic, fractured endodontically treated mandibular right first molar (tooth No. 30). The tooth had gross caries and a primary periodontal abscess with secondary endodontic involvement. Extensive periapical pathology was present on the mesial roots, and all roots showed extensive external resorption. The tooth was deemed nonrestorable.
In May 2011 the tooth was extracted using local infiltration anesthesia and IV conscious sedation, and the socket was grafted with a cortical and cancellous particulate bone allograft (Puros®, Zimmer Dental) enhanced with platelet-derived growth factor. A resorbable collagen membrane with signaling growth factors (DynaMatrix®, Keystone Dental, Inc) was placed prior to primary closure. Figure 41 shows a radiographic view of the grafted position No. 30, 8 months later.
In February 2012 the patient enrolled in the multicenter study, and a 5.5-mm x 11.5-mm implant (Genesis) was placed (Figure 42). Minor contour bone grafting of the site was also performed using autogenous bone directly against the buccal cortex and layered thereafter with a corticocancellous allograft (Puros) along the lateral aspect of the implant. This was done to increase the peri-implant bone and mucosal thickness (existing bone thickness was <1 mm on the buccal aspect) in an effort to improve parameters that would reduce the incidence of recession over the long term.
The 5.5-mm implant platform was used to accommodate the high occlusal load typically associated with the molar area and to optimize the esthetic emergence profile of the final restoration, neither of which would have been feasible with a narrower implant, even in the presence of more robust socket augmentation. A healing abutment was placed (Figure 43).
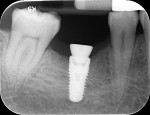
A high implant stability quotient (Osstell® ISQ = 80) (Osstell, www.osstell.com) and insertion torque values >50 Ncm allowed the prosthetic phase to begin in April 2012 (Visit 3). Figure 44 shows the periapical radiograph at Visit 3; Figure 45 shows good healing and tissue tone at this visit as well.
The final open-tray polyvinylsiloxane impression (Aquasil®, DENTSPLY International, Inc, www.dentsply.com) was also obtained at Visit 3. The impression coping was radiographically verified for accurate seating. The fixture analog was placed into the laboratory model, and a stock abutment (Genesis) was used by the laboratory to create a PFM crown with a screw-access hole in its occlusal surface (Figure 46).
In the prosthetic phase, the restorative dentist chose to use an indirect cementation technique to minimize the risk of cement entrapment/sepsis if the crown were to be cemented intraorally. In August 2012 the abutment and crown were tried in and, once proper seating and fit had been verified radiographically (Figure 47), the abutment was removed and the crown cemented extraorally with resin-modified glass-ionomer cement (FujiCem™, GC America, www.gcamerica.com). After removal of excess cement, the abutment–crown unit was then seated intraorally and torqued into the implant fixture to 35 Ncm using the standard platform-switched connection for this stock abutment. The access hole was sealed with Teflon tape and, after etching with 9.5% hydrofluoric acid, filled with nanohybrid composite resin (Renamel® NANO™, Cosmedent, www.cosmedent.com). The crown was then contoured, adjusted, and polished.
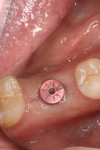
Figure 47 shows the stock abutment connected to the implant prior to extraoral cementation to the final PFM crown, which was followed by screw-retained placement (Visit 4). Figure 48 shows a good emergence profile, good gingival health, and a PD of 1 mm approximately 3 weeks after cementation (6-month follow-up), and Figure 49 confirms excellent esthetics and good occlusion at the 18-month follow-up visit (Visit 7).
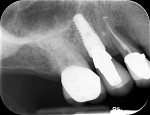
Figure 50 shows the final periapical view of the osseointegrated implant, abutment, and final screw-retained PFM crown at the 18-month follow-up (Visit 7). Bone loss of 1 mm to 2 mm is radiographically apparent around the implant in this view, as compared to 2 months post-implant placement (Figure 44). However, this is likely of little or no clinical significance, because no PD recorded at 18 months exceeded 3 mm (Table 6). The process by which this bone loss probably occurred is discussed below.
Discussion
Maintenance of marginal bone levels and soft-tissue dimensions has been encouraging in this first multicenter clinical study of this biomimetic pink implant system. The five cases presented here provide further clinical evidence of hard- and soft-tissue stability associated with this system according to the proscribed study endpoints, as well as good gingival health and excellent gingival/restorative esthetics. All cases in this multicenter study are currently beyond 3 years after final crown placement. This article (and its companion case series by Murphy et al published in 201611) present the first published data from this 5-year prospective study.
The two most extensively reported variables in the literature for osseointegrated implant placement are type of placement51-55 and platform switching.34,37,39,56-60 Both of these variables were well represented in these two series. Three of the five cases presented here were immediate implant placements; the remaining two were staged pending post-extraction graft integration. All cases incorporated the standard platform-switch range (0.50 mm to 1.38 mm) routinely used for this implant system. All received some combination of autografting, allografting, and/or xenografting. This implant system was associated with good preservation of hard and soft tissues in the presence of all of these variables.
A systematic review and meta-analysis by den Hartog et al61 identified no differences in survival or specific marginal bone metric outcomes among immediate or delayed placement. Importantly, the authors emphasized that patient-satisfaction and soft-tissue assessments were underrepresented in studies that qualified for inclusion in their analysis. Based on post-treatment surveys, patient-satisfaction levels with outcomes in the current case series were uniformly high.
The recent meta-analysis by Kinaia et al24 identified lesser degrees of crestal bone loss in association with both immediate placement and platform switching. Other systematic reviews by Herekar et al34 and Atieh et al37 report a peri-implant soft-tissue preservation effect for platform switching as well.
The hard- and soft-tissue assessments observed in this case series are consistent with these analyses. Facial and lingual gingival height was decreased by 0.1 mm to 0.5 mm among subjects who received immediate implants (Table 6), which suggests good soft-tissue stability. Facial and lingual PDs at 18 months were ≤3.5 mm across all cases. Similarly, the range of observed stability of alveolar crestal bone among these cases displayed consistency (Table 7; series range at Visit 7, 0 to -1.75 mm for mesial and -0.2 mm to -2.04 mm for distal marginal bone levels; the previously reported three-patient series range for this study at Visit 7 was -1.1 mm to 0.4 mm11).
Not unexpectedly, changes in interproximal marginal bone-to-implant distances between implant placement and final restoration (Visits 2 and 4, respectively) reflected minor crestal bone loss (≤ -2.03 mm). The greatest bone loss at this timepoint (-2.03 mm) was observed in Case 3, which involved the use of a xenograft bone substitute and placement of adjacent implants to replace two maxillary central incisors.
Between final restoration and 18-month follow-up (Visits 4 and 7, respectively), further bone loss of a much lesser degree was observed over all five cases (≤ -0.55 mm). Interestingly, Case 3 (which showed the greatest bone loss at Visit 4) also reflected small bone gains between Visit 4 and Visit 7 (Table 7).
Clearly, the crestal bone levels maintained 18 months postoperatively in Case 3 suggest a robust scaffolding effect provided by the xenograft material used during implant placement (Figure 25). To the authors’ knowledge, no direct clinical comparative studies have addressed differences that might be expected with xenografts versus allografts or autogenous bone. With regard to graft material and implant surface, a scanning electron microscopic study by Rocchietta et al identified no measurable differences in bone formation observed with xenografts relative to native bone, and no differences in bone apposition to oxidized versus machined implants.62
Although Case 3 showed an 18-month PD of 3.5 mm on the facial aspect of implant position No. 9 (the greatest PD recorded at this timepoint), good gingival tone and excellent esthetics were also evident at this visit (Figure 26).
Of note, for Case 2, a socket gap observed on the distal aspect at implant placement (Figure 14) underwent substantial bone fill between Visits 2 and 4 (Figure 15) and remained stable not only through 18 months but also through 3 years (Table 7 and Figure 18).
As noted in the systematic review by Cosyn et al,28 as well as in a 3-year follow-up study of immediate implant placement in the esthetic zone by Cosyn et al,26 the critical implant-esthetic outcome variable of midbuccal recession, while sometimes equivocal, is minimized in the presence of thick biotype, intact labial plate, and immediate provisionalization. This case-selection paradigm is echoed in a case series by Vinnakota et al40 and is consistent with the results observed in Case 3, in which all of these features were present or performed.
Against such a backdrop of “ideal” case selection, it should be noted that no clinical scenario presented, neither in this case series nor in the earlier one from this multicenter study,11 captured all of these criteria, and, in fact, the cases displayed a challenging range of clinical variations that probably better approximates the clinical reality of interdisciplinary esthetic implant practice. Based upon clinical observations, all of these patients had relatively thick biotypes, with facial gingival thickness ≥2 mm through which a periodontal probe could, at most timepoints, not be visualized50 (exceptions were Case 3 at Visit 2, and Case 5 at Visit 6), including the 18-month assessment (Visit 7).
It could be hypothesized that the esthetic properties of a pink implant system could have more of a critical bearing on color harmony at the gingival margin in a thin-biotype patient. Such biotype comparisons should be addressed specifically in the design of future studies of this implant system.
In addition, some subjects in this series received immediate provisionalization while others did not, and there was a range of bone grafting and regenerative interventions, all of which appeared, from a clinical and photographic standpoint, to demonstrate a uniformly pleasant and lasting esthetic impact at timepoints approaching or exceeding 3 years post-implant placement.
For Case 5, it is unlikely that high insertion torque (>50 Ncm) resulted in the observed radiographic bone loss (Figure 44 and Figure 50). Rather, this is more likely a function of the quality of the bone within the augmented socket at its most distant peripheral position from the vascular base during socket augmentation (ie, vascular base arising from the resultant lateral walls and apex). This portion may not have become fully vascularized and achieved vital bone prior to having forces applied to it. The bone loss was likely the response force application to residual graft particles and poorly vascularized bone (which explains why mechanotransduction responded in crestal loss as the greatest stress application was applied to the least integrated, least vascularized, and/or least consolidated bone outcome that failed to result in a true functional matrix). It is also possible that the bone graft had not completely resorbed at the time of implant placement and continued to solidify and condense after the implant was placed. Overall, this degree of bone loss is probably not clinically significant, as no PD recorded at 18 months exceeded 3 mm. It is possible that variations in radiographic angulation at successive visits resulted in distortion of bone level assessments; as with all subjects in this study, this case will be monitored for changes in bone level throughout the 5-year observation period. At the 18-month follow-up, crestal bone appeared stable and the grafted area surrounding the implant appeared to be very dense at the cortex (Figure 50).
Across all cases, excellent esthetic outcomes (including soft-tissue emergence profiles) were uniformly observed. In the current series, two subjects enrolled in the study with the presence or history of combined periodontic–endodontic involvement of the tooth to be replaced. One of these received an immediate implant (GC-08, tooth No. 4); the other followed a brief yet fairly aggressive regenerative course (TS-03, tooth No. 30). Both achieved excellent esthetic outcomes (Figure 9, Figure 11, Figure 49, and Figure 50; Table 6), with buccal height gains of ≥1.2 mm (Table 6). Taken together, these hard- and soft-tissue observations are consistent with good to excellent clinical outcomes and associated benchmarks in the literature. Furthermore, after 18 months’ follow-up, all cases presented here satisfy the study protocol definition for implant success (bone loss ≤3 mm; Table 7), thus demonstrating a 100% implant success rate.
This five-case series offers multiple and disparate clinical variables for the use of this implant system, resulting in satisfactory to excellent outcomes and running a gamut from two-stage implant-site development for a mandibular molar replacement (Case 5) to immediate replacements in the esthetic zone, one of which employed xenografting (Case 3) while the other required no grafting (Case 2). Given such a broad range of clinical variables across such a small number of cases, attribution of outcome to any of these factors amounts to speculation and is beyond the scope of a case-series article.
Statistical analyses of the entire study population, which are to be presented in the 3- and 5-year reports of the results of this multicenter study, will be necessary to reliably assess any significance of these and other numerical, surgical, prosthetic, and qualitative variables and associated observations presented in this case series. Suffice it to say that, based on this case series, this implant system has demonstrated clinically and esthetically acceptable results in the hands of periodontists, prosthodontists, and restorative dentists over a 3-year period, with a uniformly high degree of patient and clinician satisfaction.
Ongoing multicenter studies of esthetic implant variables for this system are addressing color metrics such as pink and white esthetic scores and quantitative reporting of patient-satisfaction survey data. Patients and clinicians can benefit from representative case series such as the one presented here, as they provide early and valuable exposure for in-progress prospective multicenter studies evaluating novel dental implant designs.
Conclusion
The novel pink implant system used in these five cases produced uniformly excellent esthetic results in terms of periodontal and restorative outcomes, as well as consistent marginal alveolar-bone and soft-tissue stability, across a variety of real-life clinical situations. In ongoing follow-up observations approaching or exceeding 3 years, the system continues to maintain consistent clinical performance and patient satisfaction and, thus, shows great promise to continue to deliver similar high-quality results spanning the diverse implant-restorative interdisciplinary spectrum.
ACKNOWLEDGMENTS
The authors thank the patients who participated in the study and allowed their cases to be presented here; William Kats, DDS, at Tower Dental, Downers Grove, Illinois; Stephen P. Lukin, DDS, at Lukin Family Dentistry, Sugar Land, Texas; and Dennis B. Hartlieb, DDS, at Chicago Beautiful Smiles, Chicago, Illinois, for execution of the restorative treatment phases for the patients described in Cases 1, 4, and 5, respectively; Keystone Dental, Inc. for conducting and providing an educational grant in support of this clinical study; and Scott A. Saunders, DDS, ELS, at Dental and Medical Writing and Editing, LLC (DMWE), Lancaster, Pennsylvania, for professional dental and medical writing and editing services in preparation of the manuscript.
DISCLOSURE
The primary investigators (Drs. Mandelaris, Murphy, Rosenfeld, Polack, and Scheyer) received an educational grant from Keystone Dental, Inc. in support of this study.
REFERENCES
1. Schwartz-Arad D, Laviv A, Levin L. Failure causes, timing, and cluster behavior: an 8-year study of dental implants. Implant Dent. 2008;17(2):200-207.
2. Paquette DW, Brodala N, Williams RC. Risk factors for endosseous dental implant failure. Dent Clin North Am. 2006;50(3):361-374, vi.
3. Rosenberg ES, Cho SC, Elian N, et al. A comparison of characteristics of implant failure and survival in periodontally compromised and periodontally healthy patients: a clinical report. Int J Oral Maxillofac Implants. 2004;19(6):873-879.
4. Perry J, Lenchewski E. Clinical performance and 5-year retrospective evaluation of Frialit-2 implants. Int J Oral Maxillofac Implants. 2004;19(6):887-891.
5. Weng D, Jacobson Z, Tarnow D, et al. A prospective multicenter clinical trial of 3i machined-surface implants: results after 6 years of follow-up. Int J Oral Maxillofac Implants. 2003;18(3):417-423.
6. Kalman L, MacIntosh K. The use of pink porcelain to manage a malposed anterior implant: case report. J Can Dent Assoc. 2013;79:d117.
7. Small BW. The use of pink porcelain for gingival defects in restorative dentistry: a case report. Gen Dent. 2010;58(4):285-287.
8. Garcia LT, Verrett RG. Metal-ceramic restorations—custom characterization with pink porcelain. Compend Contin Educ Dent. 2004;25(4):242-246.
9. McGuire MK, Scheyer T, Ho DK, et al. Esthetic outcomes in relation to implant-abutment interface design following a standardized treatment protocol in a multicenter randomized controlled trial—a cohort of 12 cases at 1-year follow-up. Int J Periodontics Restorative Dent. 2015;35(2):149-159.
10. Cooper LF, Reside G, Stanford C, et al. A multicenter randomized comparative trial of implants with different abutment interfaces to replace anterior maxillary single teeth. Int J Oral Maxillofac Implants. 2015;30(3):622-632.
11. Murphy KG, Polack MA, Arzadon JM, et al. A report of three cases from an ongoing prospective clinical study on a novel pink biomimetic implant system. Compend Contin Educ Dent. 2016;37(2):S1-S12.
12. Bertollo N, Sandrini E, Dalla Pria P, Walsh WR. Osseointegration of multiphase anodic spark deposition treated porous titanium implants in an ovine model. J Arthroplasty. 2015;30(3):484-488.
13. Sandrini E, Giordano C, Busini V, et al. Apatite formation and cellular response of a novel bioactive titanium. J Mater Sci Mater Med. 2007;18(6):1225-1237.
14. Giordano C, Chiesa R, Sandrini E, et al. Physical and biological characterizations of a novel multiphase anodic spark deposition coating to enhance implant osseointegration. J Mater Sci Mater Med. 2005;16(12):1221-1229.
15. Giordano C, Sandrini E, Del Curto B, et al. Titanium for osteointegration: Comparison between a novel biomimetic treatment and commercially exploited surfaces. J Appl Biomater Biomech. 2004;2(1):35-44.
16. Chiesa R, Sandrini E, Santin M, et al. Osteointegration of titanium and its alloys by anodic spark deposition and other electrochemical techniques: a review. J Appl Biomater Biomech. 2003;1(2):91-107.
17. Giavaresi G, Fini M, Chiesa R, et al. A novel multiphase anodic spark deposition coating for the improvement of orthopedic implant osseointegration: an experimental study in cortical bone of sheep. J Biomed Mater Res A. 2008;85(4):1022-1031.
18. Giavaresi G, Chiesa R, Fini M, Sandrini E. Effect of a multiphasic anodic spark deposition coating on the improvement of implant osseointegration in the osteopenic trabecular bone of sheep. Int J Oral Maxillofac Implants. 2008;23(4):659-668.
19. Park SE, Da Silva JD, Weber HP, Ishikawa-Nagai S. Optical phenomenon of peri-implant soft tissue. Part I. Spectrophotometric assessment of natural tooth gingiva and peri-implant mucosa. Clin Oral Implants Res. 2007;18(5):569-574.
20. Ishikawa-Nagai S, Da Silva JD, Weber HP, Park SE. Optical phenomenon of peri-implant soft tissue. Part II. Preferred implant neck color to improve soft tissue esthetics. Clin Oral Implants Res. 2007;18(5):575-580.
21. Pecnik CM, Roos M, Muff D, et al. In vitro color evaluation of esthetic coatings for metallic dental implants and implant prosthetic appliances. Clin Oral Implants Res. 2015;26(5):563-571.
22. Sumi T, Takeshita K, Takeichi T, et al. Patient-specific gingiva-colored abutments: a case series. Int J Periodontics Restorative Dent. 2014;34(4):469-475.
23. Polack MA. Restoration of maxillary incisors with an innovative biomimetic implant system: a case report. J Implant Adv Clin Dent. 2012;4(5):39-50.
24. Kinaia BM, Shah M, Neely AL, Goodis HE. Crestal bone level changes around immediately placed implants: a systematic review and meta-analyses with at least 12 months’ follow-up after functional loading. J Periodontol. 2014;85(11):1537-1548.
25. Huynh-Ba G, Meister DJ, Hoders AB, et al. Esthetic, clinical and patient-centered outcomes of immediately placed implants (Type 1) and early placed implants (Type 2): preliminary 3-month results of an ongoing randomized controlled clinical trial. Clin Oral Implants Res. 2016;27(2):241-252.
26. Cosyn J, Eghbali A, De Bruyn H, et al. Immediate single-tooth implants in the anterior maxilla: 3-year results of a case series on hard and soft tissue response and aesthetics. J Clin Periodontol. 2011;38(8):746-753.
27. Slagter KW, den Hartog L, Bakker NA, et al. Immediate placement of dental implants in the esthetic zone: a systematic review and pooled analysis. J Periodontol. 2014;85(7):e241-e250.
28. Cosyn J, Hooghe N, De Bruyn H. A systematic review on the frequency of advanced recession following single immediate implant treatment. J Clin Periodontol. 2012;39(6):582-589.
29. Calvo Guirado JL, Saez Yuguero MR, Pardo Zamora G, Muñoz Barrio E. Immediate provisionalization on a new implant design for esthetic restoration and preserving crestal bone. Implant Dent. 2007;16(2):155-164.
30. Lazzara RJ, Porter SS. Platform switching: a new concept in implant dentistry for controlling postrestorative crestal bone levels. Int J Periodontics Restorative Dent. 2006;26(1):9-17.
31. Baumgarten H, Cocchetto R, Testori T, et al. A new implant design for crestal bone preservation: initial observations and case report. Pract Proced Aesthet Dent. 2005;17(10):735-740.
32. Cappiello M, Luongo R, Di Iorio D, et al. Evaluation of peri-implant bone loss around platform-switched implants. Int J Periodontics Restorative Dent. 2008;28(4):347-355.
33. Canullo L, Goglia G, Iurlaro G, Iannello G. Short-term bone level observations associated with platform switching in immediately placed and restored single maxillary implants: a preliminary report. Int J Prosthodont. 2009;22(3):277-282.
34. Herekar M, Sethi M, Mulani S, et al. Influence of platform switching on periimplant bone loss: a systematic review and meta-analysis. Implant Dent. 2014;23(4):439-450.
35. Annibali S, Bignozzi I, Cristalli MP, et al. Peri-implant marginal bone level: a systematic review and meta-analysis of studies comparing platform switching versus conventionally restored implants. J Clin Periodontol. 2012;39(11):1097-1113.
36. Al-Nsour MM, Chan HL, Wang HL. Effect of the platform-switching technique on preservation of peri-implant marginal bone: a systematic review. Int J Oral Maxillofac Implants. 2012;27(1):138-145.
37. Atieh MA, Ibrahim HM, Atieh AH. Platform switching for marginal bone preservation around dental implants: a systematic review and meta-analysis. J Periodontol. 2010;81(10):1350-1366.
38. Prosper L, Redaelli S, Pasi M, et al. A randomized prospective multicenter trial evaluating the platform-switching technique for the prevention of postrestorative crestal bone loss. Int J Oral Maxillofac Implants. 2009;24(2):299-308.
39. Canullo L, Rasperini G. Preservation of peri-implant soft and hard tissues using platform switching of implants placed in immediate extraction sockets: a proof-of-concept study with 12- to 36-month follow-up. Int J Oral Maxillofac Implants. 2007;22(6):995-1000.
40. Vinnakota DN, Akula SR, Krishna Reddy VV, Sankar VV. A staged approach of implant placement in immediate extraction sockets for preservation of peri-implant soft and hard tissue. J Indian Soc Periodontol. 2014;18(2):267-271.
41. Dornbush JR, Reiser GM, Ho DK. Platform switching and abutment emergence profile modification on peri-implant soft tissue. Alpha Omegan. 2014;107(2):28-32.
42. Barwacz CA, Stanford CM, Diehl UA, et al. Electronic assessment of peri-implant mucosal esthetics around three implant-abutment configurations: a randomized clinical trial. Clin Oral Implants Res. 2016;27(6):707-715.
43. Zuiderveld EG, den Hartog L, Vissink A, et al. Significance of buccopalatal implant position, biotype, platform switching, and pre-implant bone augmentation on the level of the midbuccal mucosa. Int J Prosthodont. 2014;27(5):477-479.
44. Prasad DK, Shetty M, Bansal N, Hegde C. Crestal bone preservation: a review of different approaches for successful implant therapy. Indian J Dent Res. 2011;22(2):317-323.
45. De Bruyn H, Raes S, Ostman PO, Cosyn J. Immediate loading in partially and completely edentulous jaws: a review of the literature with clinical guidelines. Periodontol 2000. 2014;66(1):153-187.
46. Carr AB. Survival of short implants is improved with greater implant length, placement in the mandible compared with the maxilla, and in nonsmokers. J Evid Based Dent Pract. 2012;12(1):18-20.
47. Telleman G, Raghoebar GM, Vissink A, et al. A systematic review of the prognosis of short (<10 mm) dental implants placed in the partially edentulous patient. J Clin Periodontol. 2011;38(7):667-676.
48. Pommer B, Frantal S, Willer J, et al. Impact of dental implant length on early failure rates: a meta-analysis of observational studies. J Clin Periodontol. 2011;38(9):856-863.
49. Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol. 2007;34(7):610-617.
50. Kan JY, Morimoto T, Rungcharassaeng K, et al. Gingival biotype assessment in the esthetic zone: visual versus direct measurement. Int J Periodontics Restorative Dent. 2010;30(3):237-243.
51. van Kesteren CJ, Schoolfield J, West J, Oates T. A prospective randomized clinical study of changes in soft tissue position following immediate and delayed implant placement. Int J Oral Maxillofac Implants. 2010;25(3):562-570.
52. Guarnieri R, Belleggia F, Grande M. Immediate versus Delayed Treatment in the Anterior Maxilla Using Single Implants with a Laser-Microtextured Collar: 3-Year Results of a Case Series on Hard- and Soft-Tissue Response and Esthetics. J Prosthodont. 2016;25(2):135-145.
53. Pal US, Dhiman NK, Singh G, et al. Evaluation of implants placed immediately or delayed into extraction sites. Natl J Maxillofac Surg. 2011;2(1):54-62.
54. Schropp L, Kostopoulos L, Wenzel A, Isidor F. Clinical and radiographic performance of delayed-immediate single-tooth implant placement associated with peri-implant bone defects. A 2-year prospective, controlled, randomized follow-up report. J Clin Periodontol. 2005;32(5):480-487.
55. Schropp L, Isidor F, Kostopoulos L, Wenzel A. Patient experience of, and satisfaction with, delayed-immediate vs. delayed single-tooth implant placement. Clin Oral Implants Res. 2004;15(4):498-503.
56. Galindo-Moreno P, Leon-Cano A, Monje A, et al. Abutment height influences the effect of platform switching on peri-implant marginal bone loss. Clin Oral Implants Res. 2016;27(2):167-173.
57. Chrcanovic BR, Albrektsson T, Wennerberg A. Platform switch and dental implants: A meta-analysis. J Dent. 2015;43(6):629-646.
58. Enkling N, Jöhren P, Katsoulis J, et al. Influence of platform switching on bone-level alterations: a three-year randomized clinical trial. J Dent Res. 2013;92(12 suppl):139S-145S.
59. Enkling N, Jöhren P, Klimberg V, et al. Effect of platform switching on peri-implant bone levels: a randomized clinical trial. Clin Oral Implants Res. 2011;22(10):1185-1192.
60. Schrotenboer J, Tsao YP, Kinariwala V, Wang HL. Effect of platform switching on implant crest bone stress: a finite element analysis. Implant Dent. 2009;18(3):260-269.
61. den Hartog L, Slater JJ, Vissink A, et al. Treatment outcome of immediate, early and conventional single-tooth implants in the aesthetic zone: a systematic review to survival, bone level, soft-tissue, aesthetics and patient satisfaction. J Clin Periodontol. 2008;35(12):1073-1086.
62. Rocchietta I, Dellavia C, Nevins M, Simion M. Bone regenerated via rhPDGF-bB and a deproteinized bovine bone matrix: backscattered electron microscopic element analysis. Int J Periodontics Restorative Dent. 2007;27(6):539-545.
ABOUT THE AUTHORS
Mariano A. Polack, DDS, MS
Dental Design Drs. Polack and Olano, Gainesville, Virginia
E. Todd Scheyer, DDS, MS
Perio Health Professionals, Houston, Texas; University of Texas Health Science Center at Houston, Houston, Texas; University of Texas Health Science Center at San Antonio,
San Antonio, Texas
Kevin G. Murphy, DDS, MS
Department of Periodontics, University of Maryland, Baltimore College of Dental Surgery, Baltimore, Maryland;
Kevin G. Murphy & Associates, P.A.,
Baltimore, Maryland
Joseph M. Arzadon, MD, DDS
Northern Virginia Surgical Arts,
Arlington, Virginia
Alan L. Rosenfeld, DDS
Periodontal Medicine & Surgical Specialists, Ltd.,
Chicago, Illinois
George A. Mandelaris, DDS, MS
Periodontal Medicine & Surgical Specialists, Ltd., Chicago, Illinois; Adjunct Clinical Assistant Professor,
University of Illinois, College of Dentistry, Department of Graduate Periodontics,
Chicago, Illinois