What Is an Effective Method for Treatment of Peri-implantitis?
Paul S. Rosen, DMD, MS
Abstract: Based on a high level of success, dental implant therapy has become a preferred means of treatment for a wide variety of oral problems related to historic or impending tooth loss. Peri-implantitis is a biologic complication that can threaten the survival of dental implants. Many systematic reviews and meta-analyses that have evaluated the varying treatment options available to manage this problem suggest that dental implants have questionable to poor prognoses when the bone loss becomes moderate to advanced. This article presents two case reports that demonstrate successful treatment of peri-implantitis. A commonality in both cases was the implementation of thorough surface decontamination and ablation of soft tissues for residual bacteria or particulate matter.
Patients who have been treated with dental implants historically have demonstrated high success and survival rates.1 As a result, patients and clinicians have come to rely upon dental implants for treatment of a wide range of situations where teeth have been or are soon expected to be lost. While the use of dental implants has been established as a highly positive approach to tooth replacement, a growing number of accounts of peri-implantitis have been documented in dental literature.2
The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions defines peri-implantitis as a plaque-associated pathological condition. This problem occurs in the tissues around dental implants and is characterized by inflammation and progressive bone loss.3,4 Abrahamsson and colleagues published a series of interviews on patients referred for peri-implantitis treatment.5 In their article they described the psychological devastation caused by this problem in patients who invested heavily in implant therapy, had high expectations for its potential success, and were ultimately disappointed by the outcome.
Systematic reviews and meta-analyses pertaining to treatment for peri-implantitis generally conclude that no recommendations may be made advocating a particular technique.6,7 This is due largely to an inability to arrest the disease problem.6 There is agreement that nonsurgical efforts tend to fail, and even when using targeted antimicrobial approaches, surgery still seems to fare only marginally better.8,9 Part of the difficulty is in surface decontamination. The microgeometry of many implants being placed today is textured extending up to the platform, and this increases the rate of bone loss if the implant becomes exposed10 and makes debridement of the implant more difficult.
Advances have been made in managing the surface microbiome more effectively through the chemotherapeutic use of citric acid.11,12 In vitro studies have demonstrated that this agent, which has shown efficacy for regeneration on teeth,13 can also produce positive benefits for dental implants.14,15 The following case examples highlight the use of citric acid in conjunction with air powder abrasion with glycine in a comprehensive therapeutic approach.
Case 1
A 66-year-old woman who had received dental implants in her maxillary arch 9 years prior returned for treatment due to advanced bone loss. The implants had been placed because of tooth loss secondary to periodontal disease and shortened roots following orthodontic treatment, which she had undergone as an adult. After the restoration of the dental implants, she maintained her follow-up recall cleanings with her restorative dentist, attending maintenance visits routinely three to four times per year. The only exception to her regular visits had been 2 years prior when she underwent successful kidney removal due to a cancerous lesion. Her current health history included hypertension, diabetes, and elevated cholesterol with oral medications. All of these conditions were well controlled.
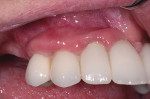
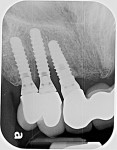
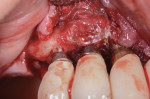
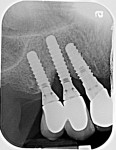
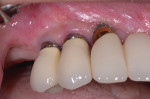
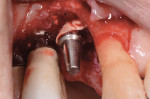
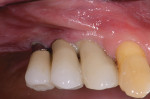
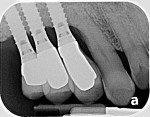
Severe inflammation and purulence on probing were noted in the area of the maxillary right first premolar, canine, and lateral incisor (Figure 1). Radiographs suggested severe peri-implantitis among these implants (Figure 2).16 A surgical course of therapy involving regeneration was chosen because of the advanced nature of the lesions and the patient's desire to maintain her dental implants.14,15
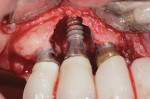
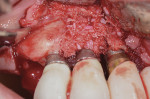
In short, sulcular full-thickness flaps were elevated on the facial and palatal aspects of this region. Bone loss was more advanced on the canine site implant and was affecting the adjacent implants (Figure 3). Surface decontamination was performed with a technique that used air powder abrasion with glycine followed by sterile water rinse, and then application of citric acid in a 50% concentration.12,14,15
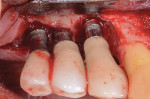
After the area was flushed with sterile water, the implant surfaces were treated with recombinant platelet-derived growth factor-BB (rhPDGF-BB) (GEM-21S®, Lynch Biologics, lynchbiologics.com), and a composite allograft consisting of mineralized and demineralized freeze-dried bone with mesenchymal cells (Osteocel®, Ace Surgical Supply Co., Inc., acesurgical.com) was placed into the lesion (Figure 4). The graft was then covered with a barrier made from amnion-chorion material (BioXclude®, Snoasis Medical, snoasismedical.com) (Figure 5), and the flap was sutured with 5-0 polytetrafluoroethylene (PTFE) (Figure 6).
The patient was placed on amoxicillin for infection control and ibuprofen for analgesia. She was instructed to use a botanical mouthrinse (PeriActive®, Straumann, straumann.com) in the first 3 months following the surgery to reduce inflammation.
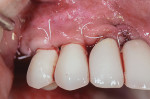
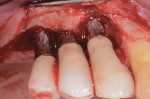
Sutures were removed at 2 weeks and the patient was continued on a 2-week interval for follow-up for the first 3 months and then monthly thereafter. At 6 months, the soft tissue appeared inflamed well beyond what the clinician had expected and hoped for (Figure 7), and a decision was made to treat the area with a neodymium-doped:yttrium aluminum garnet (Nd:YAG) laser (PerioLase® MVP-7, Millennium Dental Technologies, Inc., lanap.com) for additional soft-tissue decontamination (Figure 8). At 3 months following the laser procedure the area was noted to have healed well based on the observation of shallow probings (<4 mm) with bleeding being mostly absent and no purulence elicited.
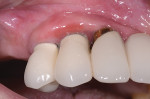
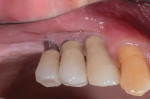
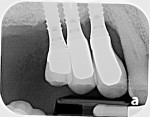
Radiographs at 3 years suggested favorable gains in hard tissue (Figure 9), and clinical evaluation showed evidence that the treatment was maintaining (Figure 10). The patient currently is attending maintenance visits on a 3-month interval.
Case 2
A 65-year-old man was referred for evaluation and treatment of moderate peri-implantitis (Figure 11 and Figure 12).16 His medical history included hypertension, for which an angiotensin-converting enzyme (ACE) inhibitor and beta blocker were taken, and diabetes that was controlled by metformin and glipizide as well as 81 mg aspirin daily. He had received dental implants in the maxillary right quadrant at the sites of both premolars and first molar approximately 8 to 9 years prior, and they had been restored with cement-retained crowns.
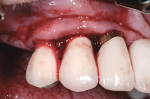
A surgical approach was used to treat the implants with full-thickness flaps extending to the maxillary right lateral incisor. Reflection of the flaps revealed that residual cement was a significant part of the etiology (Figure 13).17 Upon removal of the cement, the bony defects and implant surfaces were visualized (Figure 14) and the morphology of the lesions was determined to be class 3 for the first premolar and molar and class 2 for the second premolar.18 Because the lesion area was unable to contain graft material, a resective approach was used that included chemotherapeutic surface decontamination12 followed by implantoplasty, osteoplasty, and a second round of air powder abrasion and citric acid application (Figure 15).
The flaps were sutured using PTFE, and soft-tissue decontamination was performed using the Nd:YAG laser (Figure 16).19 Amoxicillin was used for infection control, and the patient was followed stringently in post-surgical management.
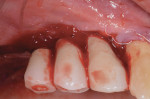
Three years later, the soft tissue had improved significantly in both color and texture, and maintainable probings of 3 mm to 4 mm were achieved (Figure 17). Periapical radiographs suggested osseous stability (Figure 18).
While the patient's oral hygiene efforts have improved, light plaque can still be found around the marginal gingival areas when the patient comes in for maintenance care every 3 to 4 months.
Discussion and Rationale for Treatment
The two case reports presented suggest that peri-implantitis can be successfully managed. The common thread to both of these cases was thorough decontamination of the surfaces of the dental implants followed by decontamination of the soft tissues using laser-assisted therapy. Air powder abrasion with glycine followed by citric acid application was used on both patients; the cases differed in the use of grafting and implantoplasty.
The ultimate objective would be to regenerate the lost bone around the dental implant, ie, achieve osseoreintegration, and maintain soft-tissue esthetics by avoiding recession in the area. Case reports have suggested that this may be possible in some instances.14,15 However, patients often present for treatment of peri-implantitis with lesions that will not permit graft containment. Implantoplasty perhaps may then be considered, as it has demonstrated favorable results.20 Based on experience, the author advocates decontaminating the dental implant's surface with air powder abrasion and citric acid prior to the implantoplasty. This physical/chemotherapeutic treatment will aid in surface decontamination, which is a major concern due to contaminated debris being released in the process of this therapy. Performing this treatment again after the implantoplasty offers the potential benefit of creating a surface that is optimal for cellular attachment.21 The hope is that readhesion of the soft tissue to the decontaminated implant surface will occur, creating a hemidesmosomal seal from the oral environment.
Peri-implantitis historically has been viewed as strictly a bacterially associated disease. More recently, however, a growing body of literature has suggested there may be more than a causal relationship between the granular titanium material found in the soft tissue surrounding the failing dental implant and the bone loss around the implant.22 A recent critical review by Mombelli and colleagues stated that there is insufficient evidence to prove a unidirectional causal relationship.22 However, the poor results of treatment, particularly those directed toward peri-implant mucositis,23 would suggest that bacteria is not the only causal agent. The source of the granular titanium may be any number of initiators ranging from insertion of the dental implant, to micromotion that occurs between the implant and its abutment, to the performing of maintenance procedures.22
Suárez-López del Amo and colleagues examined the impact of this titanium debris on the oral epithelium and determined that these particles/debris could contribute to the disruption of epithelial homeostasis and potentially compromise the oral epithelial barrier because of the damage the debris causes to the cellular DNA.24 Hence, the utilization of a laser such as the Nd:YAG may assist in removing this debris from the tissue through ablation, thereby eliminating a reservoir that could reinitiate inflammation and further breakdown.
Conclusion
Peri-implantitis traditionally has been considered a biologic complication that may lead to eventual loss of the dental implant, and approaches to care have been unable to predictably arrest this disease. It seems that peri-implantitis has been somewhat misunderstood in its etiologies, and, therefore, treatment results historically have been disappointing.
Although the two cases presented describe peri-implantitis treatments that involved varying approaches, they both utilized thorough decontamination of the implant surfaces with citric acid and air powder abrasion along with decontamination of the soft tissues of suspected debris with an Nd:YAG laser. Controlled prospective studies need to be performed to determine if these positive approaches to care based on a better understanding of disease initiators can be translated to a larger cohort of patients, potentially giving clinicians the hope that peri-implantitis can be effectively treated.
Disclosure
Dr. Rosen is a shareholder of and consultant to Snoasis Medical.
About the Author
Paul S. Rosen, DMD, MS
Clinical Professor, Division of Periodontics, Department of Advanced Oral Sciences and Therapeutics, University of Maryland School of Dentistry, Baltimore, Maryland;Adjunct Professor of Dental Implantology, James Cook University Dental School,Cairns, Australia; Private Practices, Yardley, Pennsylvania, and New York, New York
References:
1. Jemt T, Olsson M, Franke Stenport V. Incidence of first implant failure: a retroprospective study of 27 years of implant operations at one specialist clinic. Clin Implant Dent Relat Res. 2015;17(suppl 2):e501-e510.
2. Chackartchi T, Romanos GE, Sculean A. Soft tissue-related complications and management around dental implants. Periodontol 2000. 2019;81(1):124-138.
3. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol. 2018;89(suppl 1):S267-S290.
4. Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(suppl 1):S313-S318.
5. Abrahamsson KH, Wennström JL, Berglundh T, Abrahamsson I. Altered expectations on dental implant therapy; views of patients referred for treatment of peri-implantitis. Clin Oral Implants Res. 2017;28(4):437-442.
6. Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29(suppl):325-345.
7. Klinge B, Gustafsson A, Berglundh T. A systematic review of the effect of anti-infective therapy in the treatment of peri-implantitis. J Clin Periodontol. 2002;29(suppl 3):213-225.
8. Leonhardt Å, Berglundh T, Ericsson I, Dahlén G. Putative periodontal pathogens on titanium implants and teeth in experimental gingivitis and periodontitis in beagle dogs. Clin Oral Implants Res. 1992;3(3):112-119.
9. Charalampakis G, Leonhardt Å, Rabe P, Dahlén G. Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicenter study. Clin Oral Implants Res. 2012;23(9):1045-1054.
10. O'Mahoney A, MacNeill SR, Cobb CM. Design features that may influence bacterial plaque retention: a retrospective analysis of failed implants. Quintessence Int. 2000;31(4):249-256.
11. Gamal AY, Abdel-Ghaffar KA, Iacono VJ. A novel approach for enhanced nanoparticle-sized bone substitute adhesion to chemically treated peri-implantitis-affected implant surfaces: an in vitro proof-of-principle study. J Periodontol. 2013;84(2):239-247.
12. Rosen PS, Qari M, Froum SJ, et al. A pilot study on the efficacy of a treatment algorithm to detoxify dental implant surfaces affected by peri-implantitis. Int J Periodontics Restorative Dent. 2018;38(2):261-267.
13. Cole RT, Crigger M, Bogle G, et al. Connective tissue regeneration to periodontally diseased teeth. A histological study. J Periodontal Res. 1980;15(1):1-9.
14. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.
15. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6):857-863.
16. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533-540.
17. Quaranta A, Lim ZW, Tang J, et al. The impact of residual subgingival cement on biological complications around dental implants: a systematic review. Implant Dent. 2017;26(3):465-474.
18. Monje A, Pons R, Insua A, et al. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res. 2019;21(4):635-643.
19. Rosen PS, Froum SH, Froum SJ. A rationale for post-surgical laser use to effectively treat dental implants affected by peri-implantitis: 2 case reports. Int J Periodontics Restorative Dent. In press.
20. Romeo E, Ghisolfi M, Murgolo N, et al. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: clinical outcome. Clin Oral Implants Res. 2005;16(1):9-18.
21. Htet M, Madi M, Zakaria O, et al. Decontamination of anodized implant surface with different modalities for peri-implantitis treatment: lasers and mechanical debridement with citric acid. J Periodontol. 2016;87(8):953-961.
22. Mombelli A, Hashim D, Cionca N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin Oral Implants Res. 2018;29(suppl 18):37-53.
23. Schwarz F, Becker K, Sager M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J Clin Periodontol. 2015;42(suppl 16):S202-S213.
24. Suárez-López del Amo F, Rudek I, Wagner VP, et al. Titanium activates the DNA damage response pathway in oral epithelial cells: a pilot study. Int J Oral Maxillofac Implants. 2017;32(6):1413-1420.