Comparing the pH Change of Local Anesthetic Solutions Using Two Chairside Buffering Techniques
Jason H. Goodchild, DMD; and Mark Donaldson, PharmD
Abstract:
BACKGROUND: Alkalinization, or buffering of local anesthesia, is a well-documented technique to mitigate low pH levels of the preparations with reports indicating clinical benefits such as decreased onset time and injection pain. OBJECTIVE: Two methods for buffering local anesthetics are available to dentists: hand-mixing with 8.4% sodium bicarbonate or using the Onpharma® mixing system. This in vitro study compared the pH buffering ability of these two methods in seven commercially available dental local anesthetic preparations. METHODS: The authors prepared unbuffered and buffered samples of each local anesthetic preparation. The buffered samples were prepared to a 9:1 ratio (local anesthetic to sodium bicarbonate) using the Onpharma mixing system and by hand mixing. Sample pH levels were recorded using a pH meter. The samples were stored and retested after 3 and 7 days; pH was recorded via the same pH meter. RESULTS: Regardless of buffering technique used, the pH measurements of each sample increased. For six of the seven preparations, there was no significant difference between the buffering from the Onpharma mixing system and hand mixing. The 2% lidocaine with epinephrine 1:50,000 groups showed a significant difference in pH levels between the Onpharma mixing system and hand mixing (7.09 versus 6.90 respectively, P = .022). In all cases, pH of the buffered solutions continued to rise by day 3, and in most cases rose just slightly more by day 7. CONCLUSIONS: For all but one local anesthetic preparation, there was no significant difference between the pH buffering ability achieved by the Onpharma mixing system and hand mixing. The pH levels of all buffered samples increased over time. CLINICAL IMPLICATIONS: Dentists can buffer local anesthetic preparations by using the Onpharma mixing system or by hand mixing and achieve similar results. More research is needed to establish the proper ratio for buffering of 4% local anesthetic solutions. It is recommended to use buffered preparations immediately after mixing. The data from this study indicate a further alkalinization of the solution over time, and the impact of using stored solutions clinically is not clear.
Local anesthetic preparations are commonly packaged at an acidic pH measurement to increase shelf life and stability.1 Under normal conditions, local anesthetics exist in two forms: a charged form that is soluble in water and an uncharged, base form that is insoluble in water. The relative proportion of these two forms is dependent on the pH measurement of the solution and can be calculated using the Henderson-Hasselbalch equation.2
For solutions containing a vasoconstrictor, the pH range can be approximately 3 to 5, and it is believed that this low pH levels can contribute to slow onset and injection-site pain.3 To mitigate the effects of low pH-level local anesthetic solutions, the addition of sodium bicarbonate to buffer or alkalinize the pH measurement closer to physiologic range has been widely studied in medicine and dentistry. Because the uncharged, base form of local anesthetics is lipid soluble and will readily cross nerve membranes, alkalinization of solutions to drive the stoichiometric equation toward more uncharged local anesthetic molecules should lead to a faster, more effective, and profound local anesthesia clinically.
In two early in vitro studies, it was shown that buffering local anesthetics with sodium bicarbonate can potentiate their nerve impulse-blocking action on peripheral nerves.4,5 More recently, an in vivo study by Malamed et al6 demonstrated a statistically significant decrease in onset time when patients received buffered lidocaine with epinephrine compared with an unbuffered solution for an inferior alveolar nerve block. In that study, the time it took for 70% of participants to achieve pulpal anesthesia after administration of buffered and unbuffered lidocaine was 1:57 minutes and 8:30 minutes, respectively.
In 2010 the Food and Drug Administration approved the Onpharma® mixing system (Onpharma Inc., www.onpharma.com) for buffering of lidocaine. The mixing system consists of three parts: the Onpharma® mixing pen, the Onpharma® cartridge connector, and the Onset® Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive Solution. On the mixing pen, the desired amount of sodium bicarbonate can be selected via a numbered volume dial; the manufacturer recommends the addition of 0.18 mL for a 9:1 ratio of lidocaine to sodium bicarbonate.7
The aim of this in vitro study was to compare the pH buffering of seven commercially available local anesthetic preparations using a chairside hand-mixing technique with commercially available 8.4% sodium bicarbonate, versus the commercially available Onpharma mixing device with the Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive.
Materials and Methods
Seven commercially available local anesthetic solutions were supplied by DENTSPLY Pharmaceutical (www.dentsply.com) for pH testing (Table 1). All samples were prepared and stored in a temperature-controlled room at 23°C ± 2°C for the entire experiment. The study design involved preparation of two separate samples for each local anesthetic solution, both the unbuffered and buffered. Testing of sample pH level was accomplished using an Accumet® Research AR50 Dual Channel pH/Ion/Conductivity Meter (Fisher Scientific, www.fishersci.com) calibrated with reference solutions of pH levels of 4, 7, and 10. After each pH reading, the probe was wiped clean and the meter was recalibrated before switching to a different local anesthetic solution. Before each sample was tested, it was mixed for 10 seconds with a new mixing stick and then immediately tested.
pH Levels of Unbuffered Solutions
The seven local anesthetic solutions tested, their lot numbers, and pH measurement ranges as reported by the manufacturer are shown in Table 1. In each case, two identical samples were prepared by injecting the contents of two cartridges (3.6 mL) into a clean and dry testing vial. All samples were tested four separate times using the same meter and process described above.
Buffering Local Anesthetic Solutions Using Sodium Bicarbonate
A 50-mL vial of sodium bicarbonate (8.4% sodium bicarbonate injection, USP, Hospira Inc. [www.hospira.com], lot number 41-143-EV) was used to buffer the seven local anesthetic solutions shown in Table 1. The buffered solutions were prepared using the same 9:1 ratio of local anesthesia to 8.4% sodium bicarbonate described by Malamed et al.6 A cartridge volume of 1.8 mL was used for all calculations when preparing the samples.8,9 In each case, 0.18 mL of local anesthetic solution was removed from the cartridge using a 0.5-mL insulin syringe with a 28-gauge 0.5-inch needle (Kendall Monoject Insulin Syringe, Tyco Healthcare, www.tyco.com, lot number 027501). Using a separate unused syringe, 0.18 mL of the commercially available sodium bicarbonate was removed from the 50-mL vial and immediately injected into the local anesthetic cartridge. The buffered local anesthetic solutions were immediately injected into a clean and dry testing vial for pH testing. Each sample consisted of 3.6 mL of buffered local anesthetic solution and was tested four separate times.
Buffering Local Anesthetic Solutions Using the Onpharma Mixing System
The samples were prepared using the Onpharma Mixing Pen, Onpharma Cartridge Connectors, and Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive Solution. In all cases, the approved manufacturer mixing directions for the buffering of 2% lidocaine with epinephrine 1:100,000 were repeated for all the local anesthetic formulations tested (9:1 ratio of local anesthetic to 8.4% sodium bicarbonate).7 As per the study design, two separate 3.6-mL samples were created, and immediately after buffering were injected into a clean and dry mixing vial for pH testing. Each sample was tested four separate times.
pH Levels of Sodium Bicarbonate
Samples of the 50-mL vial of 8.4% sodium bicarbonate and the Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive Solution were created by injecting 2 mL of each solution into a clean and dry testing vial. Two sets of samples were created for each solution; the pH level of each sample was tested four separate times.
Storage and Stability of Buffered Samples
Twenty-eight samples were created during the experiment, each in a separate testing vial (14 buffered samples using the 50-mL vial of sodium bicarbonate and 14 buffered samples using the Onpharma mixing system). All samples were stored by placing a cover over the vials. Each sample was retested after 3 days and again after 7 days. In all cases, the samples were hand mixed with a new mixing stick for 10 seconds before pH testing.
Reporting of Data
The pH values for each unbuffered sample are reported as means, and the buffered samples are reported as means with standard deviation. The pH values of the sodium bicarbonate solutions are reported as a mean with standard deviation.
The means of the buffered solutions were analyzed using a Student’s t-test. All calculations were done using Excel (Microsoft Office 2010, Microsoft Corp.).
Results
The measured pH values for the seven tested local anesthetic solutions are shown in Table 2, column three. The pH levels of all of the local anesthetic solutions were within the labeled range as supplied by the manufacturer. The Xylocaine® solutions showed a higher pH reading (4.27 and 3.93) than those of the Articadent® solutions (3.62 and 3.68). The lowest pH value was for 4% Citanest with 1:200,000 epinephrine. For the solutions containing a vasoconstrictor (epinephrine), the pH level fell between 3.60 and 4.27. For the 3% Polocaine® and 4% Citanest® plain solutions, the pH value was 6.37 and 6.31, respectively.
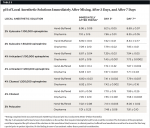
In all cases after buffering, the pH values of the local anesthetic solutions increased. With hand-buffering, the average pH value increase was 2.4 ± 0.08 and the final buffered pH level on average was 6.9 ± 0.14. Buffering each local anesthetic using the Onpharma mixing system resulted in a similar average pH level increase of 2.5 ± 0.14 and the final buffered pH value on average was 7.0 ± 0.23. Individual buffered samples are reported as means with standard deviation in columns four and five of Table 2, depending on the mixing methodology. In all cases except one, the difference in means of the buffered solutions when comparing the hand-mixed formulations to the Onpharma-mixed solutions were not significant as calculated using a Student’s t-test analysis (Table 2, column six). The one exception was the 2% Xylocaine® 1:50,000 epinephrine sample (P = .022), in which the Onpharma-mixed solution displayed a higher pH level than the hand-mixed solution.
Table 3 shows the pH level of local anesthetic solutions immediately after mixing, after 3 days, and after 7 days. In all cases, pH level of the buffered solutions continued to rise by day 3, and in most cases, rose just slightly more by day 7. Only 4% Citanest 1:200,000 epinephrine (following hand-mixing) showed a slight decrease in pH between the day 3 and day 7 recordings. The mean pH of the hand-buffered solutions at day 3 was 7.63 ± 0.29 with a mean increase from day 1 to day 3 of 0.71 ± 0.28. The mean pH value of the local anesthesia solutions using the Onpharma mixing system at day 3 was 7.56 ± 0.11 with a mean increase from day 1 to day 3 of 0.55 ± 0.09. In both cases the differences—between day-3 pH and pH increase from day 1 to day 3—were not statistically significant (P = .562 and P = .176, respectively).
The mean pH value of the hand-buffered solutions at day 7 was 8.06 ± 0.34 with a mean increase from day 3 to day 7 of 0.43 ± 0.30. The mean pH value of the local anesthesia solutions using the Onpharma mixing system was 7.82 ± 0.27 with a mean difference from day 3 to day 7 of 0.26 ± 0.23. In both cases the differences—between day-7 pH value and pH increase from day 3 to day 7—were not statistically significant (P = .169 and P = .257, respectively).
Table 4 shows the results of pH testing of sodium bicarbonate used to buffer local anesthetic solutions (50-mL vial of 8.4% sodium bicarbonate injection, USP, Hospira Inc., lot number 41-143-EV) and Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive Solution (lot numbers W0007328 and W0007361). No statistically significant difference was noted between the pH values of both of these solutions.
Discussion
The aim of this study was to compare the ability to buffer the pH values of seven commercially available local anesthetic preparations using a simple chairside hand-mixing technique with commercially available 8.4% sodium bicarbonate versus the commercially available Onpharma mixing device with the Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive. To the authors’ knowledge this is the first study to compare buffering of local anesthetics by these techniques. The literature is scant in regards to buffering articaine despite the growing popularity of this local anesthetic in clinical practice, and this is an avenue for future research. A secondary endpoint focused on the stability of the different buffered mixtures over time.
Manufacturers typically define a target pH value range at which local anesthetic preparations meet good manufacturing practices to be sold, and in this study all of the tested local anesthetic solutions, supplied by DENTSPLY Pharmaceutical, were within the appropriate ranges (Table 1). Under normal conditions local anesthetics exist in two forms: a charged form that is soluble in water and an uncharged, base form that is insoluble in water. The relative proportion of these two forms is dependent on the pH value of the solution and can be calculated using the Henderson-Hasselbalch equation.2
For solutions containing a vasoconstrictor (Xylocaine®, Articadent®, and Citanest®), the pH range can be approximately 3 to 5. Because of the ratio of charged-to-uncharged form of the local anesthetic molecule at lower pH value, the non-active, non-lipid soluble form dominates. After injection and prior to onset of anesthetic action, the body’s physiologic pH level naturally alkalinizes the local anesthetic solution, allowing for a greater proportion of the uncharged lipid soluble form. (Even at physiologic pH levels, a roughly 70:30 charged-to-uncharged ratio exists.) Therefore, low pH values may contribute to local anesthetic latency because of the time it takes the body to alkalinize low pH solutions for better local anesthetic solubility, and it may also contribute to injection-site pain because of soft-tissue injury.3,10
However, because they do not contain a vasoconstrictor and the preservative metabisulfite, plain solutions (Citanest® and Polocaine®) are available from the manufacturer at a higher pH range (closer to physiologic pH levels), which may account for their faster onset of action and less injection-site pain.11 This may also explain why both buffering techniques resulted only in a minimal pH value change following mixing with either sodium bicarbonate solution. It is unlikely, then, that buffering of plain solutions is of additional value in clinical situations.
Because the uncharged base form of local anesthetics is lipid soluble and will readily cross nerve membranes, alkalinization of solutions to drive the stoichiometric equation toward more uncharged local anesthetic molecules should lead to a faster, more effective, and profound local anesthesia clinically.1,11 Two early in vitro studies showed that buffering local anesthetics with sodium bicarbonate can potentiate their nerve impulse-blocking action on peripheral nerves.4,5 These early studies led to the development of the Onpharma Mixing Pen, Onpharma Cartridge Connectors, and Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive Solution as a convenient device for chairside mixing and delivery of buffered lidocaine.
A recent in vivo study by Malamed et al6 utilizing the Onpharma mixing system demonstrated a statistically significant decrease in onset time when patients received buffered lidocaine with epinephrine compared with an unbuffered solution. In this study, the time it took for 70% of participants to achieve pulpal anesthesia after administration of buffered and unbuffered lidocaine was 1:57 minutes and 8:30 minutes, respectively. These findings are consistent with Tortamano and colleagues12 who found mean onset of pulpal anesthesia for lidocaine with epinephrine to be 8:42 ± 3:06 minutes.
As shown in Table 2, in all samples after buffering, the pH levels of the local anesthetic solutions increased. With hand buffering, the average pH-value increase was 2.4 ± 0.08 and the final buffered pH value on average was 6.9 ± 0.14. Buffering each local anesthetic using the Onpharma mixing device resulted in a similar average pH-value increase of 2.5 ± 0.14 and the final buffered pH level on average was 7.0 ± 0.23. In both cases, the differences—between mean pH and the mean increase in pH—were not statistically significant (P = .345 and P = .129, respectively). Individual buffered samples are reported as means with standard deviation in Table 2, columns four and five, depending on the mixing methodology. In all but one of the cases, the difference in means of the buffered solutions when comparing the hand-mixed formulations to the Onpharma-mixed solutions were not significant as calculated using a Student’s t-test analysis (Table 2, column six). The one exception was the 2% Xylocaine with 1:50,000 epinephrine sample (P = .022) in which the Onpharma device was able to raise the pH level of the commercially available product from 3.93 to 7.09 ± 0.10 versus the hand-mixed formulation, which could only raise the pH value to 6.90 ± 0.10. Because this was a nonclinical study, it could not be concluded that this difference was clinically significant; however, it was a noteworthy finding.
The results of this study suggest that, in general, the proposed chairside hand-mixing methodology is as effective as the commercially available Onpharma mixing system in buffering local anesthetics with sodium bicarbonate—specifically for lidocaine, for which the Onpharma device is indicated, but also with similar results following buffering of any of the other local anesthetics tested. It is possible, too, that this hand-mixing technique is a more financially economical option.
Malamed et al6 found that raising the pH level of a cartridge of 2% lidocaine with 1:100,000 epinephrine anesthetic closer to physiologic pH level (7.3) required 0.18 mL of the Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive when utilizing the Onset mixing device. Therefore, the investigators delivered 0.18 mL of 8.4% sodium bicarbonate into the anesthetic cartridge while removing the same amount of anesthetic, resulting in a mixed ratio of 9:1. According to the Henderson-Hasselbalch equation, the ratio of uncharged (active) anesthetic molecules to charged (inactive) anesthetic molecules changes during this buffering process from 11,000:1 at a pH value of 3.5 to 3:1 at a pH level of 7.3. While this same study did not investigate articaine, it is reasonable that this solution may be less stable following a 9:1 mixing ratio because there is twice as much drug compared with lidocaine (4% solution versus 2% solution). As the authors recorded pH value changes in all seven of the solutions beyond 24 hours (Table 3), the Articadent® solutions and Citanest® with epinephrine 1:200,000 began to form a precipitate by day 3, perhaps due to too much of the uncharged base form being created. Further studies should focus on the proper ratio of local anesthetic and 8.4% sodium bicarbonate for 4% solutions because there could be clinical implications, and perhaps solutions of higher concentrations such as those containing articaine and prilocaine would be more desirable if buffered at a more diluted ratio. By day 7, all samples had formed a layer of precipitate on the surface, which required mixing before pH levels could be recorded.
Another consequence of buffering local anesthetics with sodium bicarbonate, which relates to stability of the solution, is the production of carbon dioxide (CO2) as a byproduct. Catchlove13 first demonstrated that CO2 in a lidocaine solution has an independent anesthetic effect and that both chemicals have similar effects on peripheral nerves. He suggested that in situations in which a solution contains both lidocaine and CO2, the CO2 may cause the more immediate form of analgesia because it diffuses rapidly through the nerve sheath and probably reaches the axon before the local anesthetic. While this initial effect may be beneficial, as a gas, however, buffered anesthetics in a glass carpule may be considered unstable. Without the timely injection of the buffered mixture, the unreleased gas may be further responsible for the recognized precipitate over time. Tissue damage from such an unstable mixture and precipitate could also be of clinical concern.
Onpharma instructs that buffered solutions be used immediately; premixing and storage is not recommended. Bartfield and colleagues14 studied whether there were differences in pain at injection, pH levels, and percentage lidocaine between lidocaine and sodium bicarbonate-buffered lidocaine. They recorded data immediately after mixing, at 1 day, and again at 7 days. Besides an approximately 10% decrease in lidocaine concentration, the authors found no statistically significant difference in pain at injection or pH values from freshly mixed to 7 days. All buffered solutions were significantly less painful than unbuffered lidocaine. Robinson et al15 studied the stability of sodium bicarbonate-buffered local anesthetic solutions containing epinephrine. The pH of the buffered solutions decreased slightly over the 24-hour period following buffering, however, the epinephrine concentration significantly decreased in all the buffered solutions. In the buffered lidocaine and buffered bupivacaine groups, the epinephrine concentrations decreased by 100%. It was the authors’ conclusion that buffered local anesthetic solutions containing epinephrine should not be premixed and stored prior to clinical use.
In the present authors’ study, the pH values of the buffered local anesthetic solutions increased at 3 days and in most cases increased further by day 7. The average pH value of the buffered solutions at 3 days was 7.63 ± 0.29 after hand mixing and 7.56 ± 0.11 after using the Onpharma mixing system. At 7 days, the average pH value of the buffered solutions was 8.06 ± 0.34 after hand-mixing and 7.82 ± 0.27 after using the Onpharma mixing system. The present study did not include concentration testing for lidocaine or epinephrine. Based on the studies by Bartfield et al14 and Robinson et al,15 it is reasonable to presume lidocaine concentrations remained stable and epinephrine concentrations decreased.
In trying to control for potential confounders, pH testing of both sodium bicarbonate formulations was performed (Table 4) without any statistically significant difference being recorded (8.12 ± 0.13 versus 8.11 ± 0.11 for the 50-mL vial of 8.4% sodium bicarbonate injection, USP and the 8.4% Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive Solution, respectively). These near-identical results helped control for at least one variable in this in vitro study; however, the authors do acknowledge other study limitations. Primarily, this was a laboratory investigation and, as such, clinical implications can be suggested only as a direction of research for future clinical trials. The total of 28 samples were rigorously controlled for any aseptic, temperature, humidity, or ultraviolet light-exposure excursion; however, this study is based on just these 28 samples using a single laboratory site.
Conclusions
This in vitro study is the first to compare the pH buffering of seven commercially available local anesthetic preparations using a simple chairside hand-mixing technique with commercially available 8.4% sodium bicarbonate, versus the commercially available Onpharma mixing device with the Onset Sodium Bicarbonate Injection, 8.4% USP Neutralizing Additive. Both techniques were equally effective in raising the pH levels of all seven of the commercially available local anesthetics tested despite the Onpharma product having only been approved for buffering lidocaine.
While these results may have some significant clinical implications, additional clinical studies are needed to assess the safety and efficacy of 4% local anesthetic solutions. Additionally, the use of stored buffered solutions is not recommended and dentists should use alkalinized local anesthetic as close to mixing time as possible. Over time, the pH levels of the buffered solutions may continue to rise and, in some cases, a precipitate may form. Furthermore, the production of CO2 as a byproduct of the buffering reaction, while beneficial initially, may result in further instability of the mixture over time.
Acknowledgment
The authors would like to thank lab technician Vilma Torres for her help in testing and storing samples.
Disclosures
DENTSPLY Pharmaceutical, York, PA, donated the local anesthetics solutions used in this study. The authors received no financial assistance or remuneration for the completion of this study.
About the Authors
Jason H. Goodchild, DMD
Clinical Education Manager
Focus North America
Clinical Affairs, Dentsply Sirona Inc.
Milford, Delaware
Mark Donaldson, PharmD
Director
Clinical Pharmacy Performance Services
Vizient Inc.
Whitefish, Montana
Clinical Professor
Department of Pharmacy
University of Montana
Missoula, Montana
Clinical Associate Professor
School of Dentistry
Oregon Health & Science University
Portland, Oregon
References
1. Christoph RA, Buchanan J, Begaila K, Schwartz S. Pain reduction in local anesthetic administration through pH buffering. Ann Emerg Med. 1988;17(2):117-120.
2. Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59(2):90-101.
3. Whitcomb M, Drum M, Reader A, et al. A prospective, randomized, double-blind study of the anesthetic efficacy of sodium bicarbonate buffered 2% lidocaine with 1:100,000 epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2010;57(2):59-66.
4. Gissen AJ, Covino BG, Gregus J. Differential sensitivity of fast and slow fibers in mammalian nerve. IV. effect of carbonation of local anesthetics. Reg Anesth. 1985;10(2):68-75.
5. Wong K, Stricharzt GR, Raymond SA. On the mechanisms of potentiation of local anesthetics by bicarbonate buffer: drug structure-activity studies on isolated peripheral nerve. Anesth Analg. 1993;76(1):131-143.
6. Malamed SF, Tavana S, Falkel M. Faster onset and more comfortable injection with alkalinized 2% lidocaine with epinephrine 1:100,000. Compend Contin Educ Dent. 2013;34 spec no 1:10-20.
7. Onpharma® Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution [package insert]. Los Gatos, CA: Onpharma Inc; 2011. http://www.onpharma.com/pdf/Onpharma_Sodium%20Bicarbonate_Additive_Solution_Package_Insert.pdf. Accessed March 31, 2016.
8. Robertson D, Nusstein J, Reader A, et al. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007;138(8):1104-1112.
9. Malamed SF. Clinical action of specific agents. In: Handbook of Local Anesthesia. 6th ed. St. Louis, MO: Elsevier Mosby; 2014:56.
10. Wahl MJ, Scmitt MM, Overton DA. Injection pain of prilocaine plain, mepivacaine plain, articaine with epinephrine, and lidocaine with epinephrine. Gen Dent. 2006;54(3):168-171.
11. Malamed SF. Buffering local anesthesics in dentistry. The Pulse. 2011;44(1):7-9.
12. Tortamano IP, Siviero M, Lee S, et al. Onset and duration period of pulpal anesthesia or articaine and lidocaine in inferior alveolar nerve block. Braz Dent J. 2013;24(4):371-374.
13. Catchlove RF. The influence of CO2 and pH on local anesthetic action. J Pharmacol Exp Ther. 1972;181(2):298-309.
14. Bartfield JM, Homer PJ, Ford DT, Sternklar P. Buffered lidocaine and a local anesthetic: an investigation of shelf life. Ann Emerg Med. 1992;21(1):16-19.
15. Robinson J, Fernando R, Sun Wai WY, Reynolds F. Chemical stability of bupivacaine, lidocaine and epinephrine in pH-adjusted solutions. Anaesthesia. 2000;55(9):853-858.