Periodontitis and the End-Stage Renal Disease Patient Receiving Hemodialysis Maintenance Therapy
Ronald G. Craig, DMD, PhD; and Peter Kotanko, MD
Abstract
Atherosclerotic complications, including myocardial infarction and stroke, are highly prevalent and associated with increased systemic inflammation in patients who have end-stage renal disease (ESRD) and are receiving renal hemodialysis maintenance therapy. In the general population, an increasing body of evidence suggests periodontitis can contribute to systemic inflammation and may contribute to atherosclerotic complications. In addition, results of recent interventional trials suggest effective periodontal therapy may decrease systemic inflammation as well as endothelial dysfunction, an early predictor of atherosclerotic complications. Because moderate-to-severe periodontitis appears to be highly prevalent in the renal hemodialysis population, effective periodontal therapy may reduce systemic inflammation and thereby become a treatment consideration for this population. This article will acquaint dental practitioners with ESRD and the association between systemic inflammation and mortality. Also discussed are the possible contributions of destructive periodontal diseases to systemic inflammation and the dental management of patients receiving renal replacement therapies.
The dental profession will be asked to provide care for increasing numbers of patients receiving renal replacement therapies. This is partly because of the increasing prevalence of end-stage renal disease (ESRD) in industrialized nations and recent advances in renal replacement technologies, which have prolonged life expectancies. In addition, several studies have suggested that periodontitis is especially prevalent and severe in patients receiving renal hemodialysis,1 the most common form of renal replacement therapy. Systemic inflammation is highly prevalent within the renal hemodialysis population and closely associated with atherosclerotic complications such as myocardial infarction and stroke, which are the most frequent causes of mortality in these patients.2 In the general population, an expanding body of evidence has shown periodontitis to contribute to systemic inflammation and may also play a role in atherosclerotic complications, such as myocardial infarction and stroke. In addition, recent interventional studies have suggested aggressive periodontal therapy may decrease serum markers of inflammation as well as endothelial dysfunction, an early vascular event common to several chronic conditions, including atherosclerosis, diabetic complications, and possibly some forms of chronic renal disease. Therefore, it may be possible that effective periodontal therapy may be shown to decrease systemic inflammation in renal hemodialysis populations and thereby become a treatment consideration in the future.
Because of the medical complexity of patients receiving renal hemodialysis and in anticipation of the increased role that dentistry may play in their healthcare management, this article will acquaint dental practitioners with ESRD and the association between systemic inflammation and mortality in these patients. Also discussed are the possible contributions of periodontitis to systemic inflammation and the dental management of patients receiving renal replacement therapy.
ESRD and Renal Replacement Therapies
The kidneys perform four essential and diverse functions: excretion and metabolism of metabolic end products (eg, urea), regulation of blood fluid volume and electrolyte concentrations, regulation of erythrocyte production in the bone marrow through secretion of erythropoietin, and calcium homeostasis through hydroxylation of vitamin D3 into active or inactive metabolites.3 Therefore, replacement of lost kidney function for patients with ESRD requires extensive medical intervention. Renal function is, in part, assessed by measurement of the glomerular filtration rate (GFR). Normal adult GFR varies between 100 mL min-1/1.73 m2 and 120 mL min-1/1.73 m2 body surface area. With the progression of glomerular or interstitial renal disease, kidney function decreases, which leads to the retention of a large number of toxic compounds that are normally cleared by the kidney, collectively termed the uremic syndrome.4 Disturbances in blood electrolytes (hyperkalemia, hyperphosphatemia); acid/base balance (metabolic acidosis); sodium retention and consecutive increased blood volume (hypertension); anemia; and renal osteodystrophy also develop. In early chronic renal disease, the remaining functioning kidney glomerulae compensate by hyperfiltration and hypertrophy. If chronic kidney disease is detected early, additional interventional strategies to maintain homeostasis can be used, including diet modifications and use of phosphorus-binding compounds, 1,25-dihydroxyvitamin D3, recombinant human erythropoietin, and antihypertensive medications. However, once the GFR falls below 10 mL min-1/1.73 m2 to 20 mL min-1/1.73 m2 body surface area and blood urea nitrogen levels rise above 100 mg/dL to 150 mg/dL, renal compensatory mechanisms fail and ESRD ensues.5
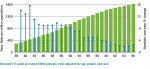
The most common causes of ESRD, in order of prevalence, are diabetes mellitus, hypertension, glomerulonephritis, and cystic kidney disease. Approximately 42% of patients receiving renal dialysis replacement therapy also have type 2 diabetes. In 2006 the incidence in the US of new ESRD cases was 360 per million individuals and 327,754 patients were receiving renal hemodialysis maintenance therapy.6 Annually, the number of patients given a diagnosis of ESRD in the US has been increasing (Figure 1). This has been mostly attributed to the rising prevalence of type 2 diabetes.
ESRD is fatal without renal replacement therapy, which can be provided by renal dialysis, peritoneal dialysis, or kidney transplantation.5 Renal hemodialysis is the most common form of renal replacement therapy in adults with ESRD. Renal hemodialysis typically employs a surgically created arteriovenous fistulae, usually sited in the forearm, to access the patient’s circulatory system. Using wide-bore needles, blood is then passed through a hemodialysis machine that uses a membrane to clean the blood from low-molecular-weight substances via a diffusion process against a large volume (120 L to 150 L per dialysis treatment) of a pH- and electrolyte-balanced dialysis solution. Clotting during the hemodialysis session is inhibited by heparin. Renal hemodialysis sessions typically last for 3 to 5 hours and are conducted three times per week at specialized hemodialysis units.
Peritoneal dialysis uses the patient’s peritoneal cavity to dialyze urea and other small-molecular-weight compounds. Access is provided by a surgically implanted catheter through which a sterile dialysis fluid is intermittently infused and drained. Peritoneal dialysis can be performed throughout the day (continuous ambulatory dialysis) or overnight using an automated machine (automated continuous cyclic peritoneal dialysis). Peritoneal dialysis obviates the need for the patient to travel to a specialized regional dialysis unit because it can be performed in the home. However, peritoneal dialysis presents the additional risk of difficult-to-manage peritoneal infections. At best, both renal hemodialysis and peritoneal dialysis are able to provide approximately 10% of the renal function of a normal kidney. As a consequence, a patient receiving dialysis remains in a continuous state of chronic renal failure and accompanying uremic syndrome (Table 1).
Far greater renal function is provided by renal transplantation. Successful renal transplantation is dependent on closely matching the patient and donor ABO blood type and major human histocompatiblity leukocyte antigen (HLA) complex. In the absence of an identical twin, achieving an identical match of HLA complexes is nearly impossible, so immune suppression of transplant recipients is required to prevent graft rejection.7 Immune suppression is usually provided by the combination of corticosteroids; calcineurin inhibitors, such as cyclosporine A or tacrolimus, to suppress interleukin 2 production; and lymphocyte proliferation inhibitors, such as azathioprine or myco-phenolate mofetil (CellCept®, Hoffmann-La Roche Inc., Nutley, NJ). Graft survival rates of 83% for 1 year and 65% for 5 years have been reported for cadaver donor kidneys with an increase of about 10% to 15% for each period for live donor kidneys.7 Disadvantages for kidney transplantation include constant immune suppression with increased susceptibility to opportunistic infections, decreased kidney function with transplant age, and hypertension.7
Systemic Inflammation and Cardiovascular Disease in Renal Hemodialysis Patients
Patients who have ESRD and are receiving renal hemodialysis face a greatly increased rate of mortality when compared to the general population, especially among the younger age groups. In 2006 the point prevalent annual rate of mortality for renal hemodialysis populations in the US was 220.5 deaths per 1000 patients, or 22%.6 Atherosclerotic complications including myocardial infarction, cardiac arrest, cardiac arrhythmia, and stroke accounted for 44% of all reported deaths. In that year, infection was the second most common cause of death.6 Mortality is highly associated with increased systemic inflammatory burden because C-reactive protein (CRP), an acute phase protein and systemic marker of inflammatory status, is a major risk factor for both cardiac and all-cause mortality in those receiving renal hemodialysis.2 In the general population, CRP has been identified with other acute phase reactants as a major risk factor for atherosclerotic complications8-12 and supplements more traditional atherosclerotic risk factors, such as serum lipoprotein profiles, as a predictor of cardiac events.13
Prevalence of Periodontal Diseases in Renal Hemodialysis Populations
Chronic renal disease has been associated with several oral problems, including xerostomia, delayed tooth eruption, pulpal calcifications, enamel hypoplasia, decreased caries, and altered salivary pH levels.14,15 In addition, most studies have reported poor oral hygiene as reflected in increased plaque,16-20 calculus formation,14,16,17,19,21 and gingival inflammation.14,15,17,19,20 Several hypotheses have been forwarded to account for these findings. First, patients receiving renal hemodialysis are in a state of chronic renal failure, resulting in the uremic syndrome. The uremic syndrome, in turn, has been associated with immune dysfunction, including defects in both lymphocyte and monocyte function22 that could permit the overgrowth of periodontopathic bacteria. Second, nearly half the patients receiving renal hemodialysis also have type 2 diabetes.6 Historically, a strong correlation between diabetes and periodontitis incidence and severity has been reported in the general population.23 Third, the extreme psychologic and time demands that renal hemodialysis imposes on patients with ESRD may decrease the priority of maintaining optimal oral hygiene and obtaining professional dental care. In support of this possibility, several studies have reported decreased use of dental services in renal hemodialysis populations.17,20
In accord with reports of increased plaque, calculus, and gingival inflammation, there have been reports of an increased prevalence and severity of periodontitis in renal hemodialysis populations. A study of 128 patients receiving renal hemodialysis in Taiwan reported an increased level of periodontal disease as measured by the Community Periodontal Index.24 A Turkish study of 342 patients receiving renal hemodialysis reported increased prevalence and severity of periodontitis as measured by the Community Periodontal Index of Treatment Needs (CPITN).25 Another report from Taiwan of 253 patients receiving renal hemodialysis therapy found increased levels of periodontitis when compared with national data for the Taiwanese population. In this study, the best predictors for periodontitis after multiple regression analysis in the hemodialysis population were increased age, smoking, dialysis duration, and decreased levels of serum albumin.19 A 2007 study from Poland measured the gingival index, plaque index, papillary bleeding index, clinical attachment level, and CPITN in 162 participants: 35 receiving renal hemodialysis, 33 receiving continuous ambulatory peritoneal dialysis, 38 with predialysis chronic renal disease, 26 with chronic periodontitis, and 30 healthy controls. The researchers reported the incidence and severity of periodontitis to increase from health to predialysis to peritoneal dialysis to renal hemodialysis groups.26 Finally, a study of 154 patients receiving renal hemodialysis regularly visiting units in North Carolina and New York City reported greatly increased prevalence and severity of periodontitis as measured by probing pocket depth and loss of clinical attachment when compared to the 7447 dentate participants who were part of the Third National Health and Nutrition Examination Survey (NHANES III).27
However, not all studies have reported increased prevalence of periodontitis in renal hemodialysis populations. A case control study from Spain of 52 renal hemodialysis patients reported no increase in periodontitis incidence or severity but did report increased levels of periodontopathic bacteria in the hemodialysis group.18 A study from the Netherlands of 42 patients with ESRD, 28 of whom were receiving renal hemodialysis, found no increase in periodontitis incidence or severity when compared to 42 controls.21 A Turkish study of 76 patients on hemodialysis compared to 61 controls reported no difference in periodontal or dental health but did find a worsening of periodontal status with time on renal dialysis therapy.28 A follow-up study from the same group compared the periodontal status of 75 patients receiving peritoneal dialysis with 41 patients using hemodialysis and 61 controls. They found no difference in probing pocket depth; however, levels of plaque, gingival inflammation, and calculus were worse in the dialysis groups.29 Differences in study design, sample size, and methods used to measure periodontal status may account for differences in periodontal disease prevalence in renal dialysis populations.
Periodontal Diseases are Associated with Atherosclerotic Complications
The presence of periodontitis in renal hemodialysis populations may have significant bearing on hemodialysis maintenance therapy because moderate-to-severe periodontitis has been shown to contribute to systemic inflammation, including CRP, in the general population. An analysis of NHANES III found a positive association between CRP and periodontitis,30 which was also supported by results of the Atherosclerosis Risk in Communities (ARIC) study that involved 5552 participants.31 Severe periodontitis has also been associated with hyperglycemia and alteration of serum lipids, consistent with an acute phase response.32 Additional support that destructive periodontal diseases can contribute to systemic inflammation arises from studies in which elimination of periodontal infections or effective periodontal therapy has been shown to decrease markers of systemic inflammation. Full-mouth extraction in those with severe periodontal disease lowered levels of CRP, plasminogen activator inhibitor-1, and fibrinogen, all components of the acute phase response.33 Furthermore, the results of two interventional studies using aggressive, nonsurgical conventional periodontal therapy augmented with locally applied antibiotics in those with moderate-to-severe periodontitis lowered CRP levels in those who were responsive to periodontal therapy.34,35 Aggressive, nonsurgical periodontal therapy also has been shown to decrease endothelial dysfunction,36,37 a systemic vascular disorder characterized by reduced bioavailability of locally active vasodilators, such as nitric oxide and an abundance of vasoconstrictors produced by endothelial cells. Endothelial dysfunction has been shown to be an early marker for atherosclerotic complications, reflecting overall systemic inflammatory burden, genetic predisposition, and possibly additional unknown variables.38 Furthermore, periodontitis has been associated with an increased, although apparently modest, risk of atherosclerotic complications. A meta-analysis of five prospective studies with follow-up > 6 years involving 86,092 people reported those with periodontitis had a 1.14 (confidence interval 1.01 to 1.2) greater relative risk of developing coronary heart disease than periodontally healthy controls.39
In the renal hemodialysis population as well, periodontal disease has been shown to contribute to systemic inflammation and possibly increased risk for atherosclerotic complications. Elevation of CRP levels has been demonstrated to be associated with elevated serum immunoglobulin G (IgG) antibody to Porphymonas gingivalis but not serum IgG antibody to five other periodontal pathogenic bacterial species.40 A study of 253 patients receiving renal hemodialysis found severity of periodontitis to correlate with CRP levels and was inversely related to serum albumin, an acute phase protein and a marker of nutrition in hemodialysis populations.19 Decreased serum albumin was also associated with severity of periodontitis in renal hemodialysis populations in New York and North Carolina; however, CRP was not found to correlate with severity of periodontitis.41 In an 18-month follow-up report of the same population, patients with severe periodontal disease had an increased rate of death from cardiovascular events compared to those with mild or no periodontitis. This association remained after adjustment for age, gender, dialysis center and vintage, smoking status, diabetes, or hypertension.42
Several studies have suggested increased systemic inflammation from periodontitis may contribute to chronic renal disease. A cross-sectional evaluation of the 5537 participants in the ARIC study was performed to evaluate periodontal disease severity with renal insufficiency as defined as a GFR less than 60 mL min-1/1.73 m2 body surface area. People with initial or severe periodontitis were twice as likely to have renal insufficiency compared to those who were periodontally healthy.43 A study of 145 community-dwelling elders from Japan found the degree of periodontal attachment loss to correlate with creatinine clearance,44 suggesting a common link between the two diseases. A recent analysis of NHANES III reported moderate-to-severe decrease in kidney function as defined by a GFR of 15 mL min-1/1.73 m2 to 59 mL min-1/1.73 m2 body surface area to correlate with periodontal disease and edentulism.45 Finally, a prospective study of Pima Indians found the presence of periodontitis to be predictive of mortality from ischemic heart disease and diabetic nephropathy46 and to be predictive of the development of overt nephropathy and ESRD in patients with type 2 diabetes.47
The clinical relevance of the above studies linking the contribution of periodontitis to systemic inflammation and atherosclerotic complications in both the general and renal hemodialysis populations is that periodontal diseases are indeed treatable and therefore may be a reversible source of systemic inflammation. However, unlike the general population, interventional studies have not been widely attempted in the renal hemodialysis population. If interventional trials are indeed successful, the elimination of periodontal infections might become an accepted component of therapy in the maintenance of the renal hemodialysis population.
Periodontal Management of Patients on Renal Dialysis Maintenance
The patient receiving renal hemodialysis maintenance therapy is medically complex and presents the dental practitioner with several challenges in the management of a periodontal condition (Table 2). Infection and medical complications requiring hospitalization frequently occur. Accordingly, close communication between the dentist and nephrologist is essential to safely treat the patient receiving renal hemodialysis and to optimize management of the periodontal condition. Overviews on the dental management of the patient with ESRD and maintained with hemodialysis have been published;15,48 therefore, only major management issues will be summarized in this article.
Because of the increased prevalence of plaque,16-20 calculus formation,14-17,19,21 and gingival inflammation14,16,17,19,20 in this population, the patient should be carefully instructed in the importance of effective oral hygiene procedures and examined at regular maintenance intervals to ensure optimal compliance. The effectiveness of regular recall and maintenance visits for periodontal patients in the general population has been well documented and may be of even more importance in the renal hemodialysis population. A primary objective of periodontal therapy is the local elimination of Gram-negative bacterial species and their products, which will also decrease systemic inflammatory burden. Because invasive dental procedures such as root planing and extractions can result in transient bacteremia, antibiotic prophylaxis before the dental appointment administered according to the American Heart Association guidelines has been recommended to protect vascular access sites. Anemia and possible clotting deficiencies should be evaluated in consultation with the patient’s physician, and dental appointments should be scheduled the day after hemodialysis sessions in view of the heparin used during hemodialysis. Because fluid retention and resulting hypertension increases during intervals between hemodialysis sessions, dental appointments should be avoided on the day of dialysis prior to the hemodialysis. Also, because of the high prevalence of hypertension in the ESRD population, care should be used with local anesthetics containing vasoconstrictors. The patient’s current vascular access site should be determined and avoided during dental treatment. For example, care should be exercised not to impinge on the site when monitoring blood pressure. The dosage and administration of drugs cleared by the kidneys may need to be altered with respect to decreased or absent kidney function. For example, care should be used in the administration of local anesthetics to minimize the total dosage because serum albumin contains a low-affinity but high-capacity binding site49 and serum albumin may be reduced in some patients receiving renal hemodialysis. Among analgesics, postoperative use of acetaminophen is considered safe but non-steroidal anti-inflammatory drugs should be avoided.49 The half life of oxycodone is prolonged in renal failure, so the dose should be reduced and the dosage interval prolonged.49 Meperidine HCl (Demerol®, Sanofi Aventis, Bridgewater, NJ), which is metabolized to normeperidine, is dependent on kidney function for elimination and should be avoided.49 Lastly, codeine and dihydrocodeine half lives are significantly prolonged; therefore, dosages and dosage intervals should be adjusted accordingly.49
Conclusion
Moderate-to-severe periodontitis and atherosclerotic complications are prevalent in the renal hemodialysis population. Periodontitis has been associated with increased markers of systemic inflammation, including elevated CRP levels, and endothelial dysfunction, an early predictor of atherosclerotic complications, in the general population. Some recent studies suggest effective periodontal therapy may decrease systemic inflammation and endothelial dysfunction. Therefore, periodontitis in renal hemodialysis populations may be a reversible source of systemic inflammation that can be managed through effective periodontal therapy. However, whether treatment of moderate-to-severe periodontitis in renal dialysis populations will result in decreased systemic inflammation and endothelial dysfunction and, more importantly, decreased incidence of atherosclerotic complications awaits the results of interceptive clinical trials in this population.
References
1. Craig RG. Interactions between chronic renal disease and periodontal disease. Oral Dis. 2008;14(1):1-7.
2. Yeun JY, Levine RA, Mantadilok V, et al. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis populations. Am J Kidney Dis. 2000;35:469-476.
3. Fogo A, Kon W. Pathophysiology of progressive chronic renal disease. In: Avner EED, Harmon WE, Niaudet P, eds. Textbook of Pediatric Nephrology. 5th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:1267-1480.
4. Vanholder R, De Smet R, Glorieux G, et al; European Uremic Toxin Work Group (EUTox). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63(5): 1934-1943.
5. Riegden S. The management of chronic and end-stage renal failure. In: Webb N, Postlewaite R, eds. Textbook of Clinical Pediatric Nephrology. 3rd ed. Oxford, England: Oxford Medical Publications; 2003:427-445.
6. US Renal Data System. 2006 Annual Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. www.usrds.org/atlas_2006.htm. Accessed March 30, 2009.
7. Seikaly M, Ho PL, Emmett L, et al. The 12th Annual Report of the North American Pediatric Renal Transplant Cooperative Study: renal transplantation from 1987 through 1998. Pediatr Transplant. 2001;5(3): 215-231.
8. Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973-976.
9. Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12): 836-843.
10. Cesari M, Penninx B, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317-2322.
11. Pearson TA, Mensah GA, Alexander RW, et al; Centers for Disease Control and Prevention and the American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499-511.
12. Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351(25):2599-2610.
13. Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557-1565.
14. Davidovich E, Schwarz Z, Davidovitch M, et al. Oral findings and periodontal status in children, adolescents and young adults suffering from renal failure. J Clin Periodontol. 2005;32(10): 1076-1082.
15. Proctor R, Kumar N, Stein A, et al. Oral and dental aspects of chronic renal failure. J Dent Res. 2005;84(3): 199-208.
16. Souza CR, Libério SA, Guerra RN, et al. Assessment of periodontal condition of kidney patients in hemodialysis. Rev Assoc Med Bras. 2005;51(5):285-289.
17. Klassen JT, Krasko BM. The dental health status of dialysis patients. J Can Dent Assoc. 2002;68(1):34-38.
18. Castillo A, Mesa F, Liébana J, et al. Periodontal and oral microbiological status of an adult population undergoing haemodialysis: a cross-sectional study. Oral Dis. 2007;13(2):198-205.
19. Chen LP, Chiang CK, Chan CP, et al. Does periodontitis reflect inflammation and malnutrition status in hemodialysis patients? Am J Kidney Dis. 2006;47(5):815-822.
20. Naugle K, Darby ML, Bauman DB, et al. The oral health status of individuals on renal dialysis. Ann Periodontol. 1998;3(1): 197-205.
21. Bots CP, Poorterman JH, Brand HS, et al. The oral health status of dentate patients with chronic renal failure undergoing dialysis therapy. Oral Dis. 2006;12(2): 176-180.
22. Cohen G, Haag-Weber M, Hörl WH. Immune dysfunction in uremia. Kidney Int Suppl. 1997;62:S79-S82.
23. Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65(3):260-267.
24. Chuang SF, Sung JM, Kuo SC, et al. Oral and dental manifestations in diabetic and nondiabetic uremic patients receiving hemodialysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(6):689-695.
25. Duran I, Erdemir EO. Periodontal treatment needs of patients with renal disease receiving haemodialysis. Int Dent J. 2004;54(5): 274-278.
26. Borawski J, WilczyÉska-Boraski M, Stokowska W, et al. The periodontal status of pre-dialysis, chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
27. Yoshino M, Craig RG, Kuhlmann MK, et al. Prevalence of periodontitis in hemodialysis (HD) patients at 2 sites. J Amer Soc Nephrol. 2005; 16:F-PO781.
28. Bayraktar G, Kurtulus I, Duraduryan A, et al. Dental and periodontal findings in hemodialysis patients. Oral Dis. 2007;13(4):393-397.
29. Bayraktar G, Kurtulus I, Kazancioglu R, et al. Evaluation of periodontal parameters in patients undergoing peritoneal dialysis or hemodialysis. Oral Dis. 2008;14(2): 185-189.
30. Slade GD, Offenbacher S, Beck JD, et al. Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000;79(1):49-57.
31. Slade GD, Ghezzi EM, Heiss G, et al. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163(10): 1172-1179.
32. Craig RG, Yip JK, So MK, et al. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74(7):1007-1016.
33. Taylor BA, Tofler GH, Carey HM, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85(1):74-78.
34. D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83(2):156-160.
35. D’Aiuto F, Nibali L, Parkar M, et al. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84(3): 269-273.
36. Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J. 2005;149(6):1050-1054.
37. Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9): 911-920.
38. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168-175.
39. Bahekar AA, Singh S, Saha S, et al. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154(5): 830-837.
40. Rahmati MA, Craig RG, Homel P, et al. Serum markers of periodontal disease status and inflammation in hemodialysis patients. Am J Kidney Dis. 2002;40(5):983-989.
41. Kshirsagar AV, Craig RG, Beck JD, et al. Severe periodontitis is associated with low serum albumin among patients on maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2007;2(2): 239-244.
42. Kshirsagar AV, Craig RG, Moss KL, et al. Periodontal disease adversely affects the survival of patients with end-stage renal disease. Kidney Int. 2009;75(7):746-751.
43. Kshirsagar AV, Moss KL, Elter JR, et al. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2005;45(4): 650-657.
44. Yoshihara A, Deguchi T, Hanada N, et al. Renal function and periodontal disease in elderly Japanese. J Periodontol. 2007;78(7): 1241-1248.
45. Fisher MA, Talyor GW, Shelton BJ, et al. Periodontal disease and other nontraditional risk factors for CKD. Amer J Kidney Dis. 2008;51(1): 45-52.
46. Saremi A, Nelson RG, Tullouch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1): 27-32.
47. Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30(2):306-311.
48. Kerr AR. Update on renal disease for the dental practitioner. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(1): 9-16.
49. Craig RG, Hunter JM. Recent developments in the perioperative management of adult patients with chronic kidney disease. Br J Anaesth. 2008;101(3):296-310.
About the Authors
Ronald G. Craig, DMD, PhD
Associate Professor
Department of Basic Sciences and Craniofacial Biology
Department of Periodontology and Implant Dentistry New York University College of Dentistry
New York, New York
Peter Kotanko, MD
Laboratory Director
Renal Research Institute LLC
New York, New York