Mechanism of Action of a Desensitizing Fluoride Toothpaste Delivering Calcium and Phosphate Ingredients in the Treatment of Dental Hypersensitivity
Andrew J. Charig, BS; Charity A. Chapin, BS; Elizabeth E. Major, BS; Stephen Thong, PhD; Anthony E. Winston, BSc
Part I: in Vitro Sem, Edx, Profilometry, and Dentin Permeability Studies
Abstract
Tooth hypersensitivity often starts in early adulthood because of gum recession and loss of the cementum covering dentin. The opened tubules in exposed dentin provide direct pathways into the pulpal cavity where the intradental nerve is housed. Hypersensitivity pain is caused by stimuli in the mouth, which induce pressure changes on the intradental nerve. Hypersensitivity can be relieved by the application of substances, such as potassium ions, which depolarize the nerves, or by the application of compositions, which seal the outside of the tubules. In a well-controlled clinical trial, a fluoride toothpaste that delivers calcium and phosphate salts to the teeth has been shown to relieve hypersensitivity. The purpose of this study was to establish this toothpaste’s mechanism of action. Multiple treatments of dentin with aqueous slurries of the toothpaste resulted in the deposition of a coherent mineral layer covering the tubules, which increasingly reduced dentin permeability. Energy dispersive X-ray analysis of the mineral layer was consistent with the presence of amorphous calcium phosphate (ACP) or calcium hydroxyapatite together with titanium dioxide and calcium sulfate. Treatment of dentin with slurries of a calcium- and phosphate-free control toothpaste or a potassium nitrate-containing competitive toothpaste deposited particles consisting of titanium dioxide and silica, respectively, and also reduced dentin permeability. Application of a slurry of the “isolated sealants” (calcium sulfate, potassium phosphate, and sodium fluoride at the same level as delivered by the toothpaste) also deposited ACP onto dentin and reduced dentin permeability, whereas potassium nitrate solution had no significant effect. In the absence of insolubles, the desensitizing toothpaste reduced dentin permeability to a greater degree than a calcium- and phosphate-free control. Taken together, the data provide strong evidence that the fluoride toothpaste delivering calcium and phosphate ions to the tooth deposits ACP onto the dentin surface. In the presence of moisture in the mouth, ACP transforms into apatitic mineral, leaving a coherent tubule-occluding layer on the surface of the dentin.
Generally, dental hypersensitivity occurs in early adulthood when the gums recede.1-6 After the layer of cementum covering the dentin is lost because of abrasion or exposure to acidic foods, the tubules are exposed, providing open passageways to the intradental nerve.1-4 According to the well-accepted hydrodynamic theory, the pain of tooth hypersensitivity results from nondamaging stimuli in the mouth that cause changes in fluid flow within dentinal tubules and pressure the nerves.1-8
Tooth hypersensitivity can be relieved by inactivating the intradental nerve and inhibiting neural transmission, using suitable medications. Alternatively, another method of treating tooth hypersensitivity is to seal the tubules, which prevents external mechanical, thermal, and osmotic stimuli from reaching the nerves.2-6,8
Potassium ions have been shown in animal studies to act directly on the nerves and to reduce sensory activity.9 However, delivering potassium ions to the nerves within the pulpal cavity against the continuous outward flow of dentinal fluid would seem somewhat problematic.1,10-12 Nevertheless, potassium ions, in the form of potassium nitrate at a 5% concentration, are widely used as the active agents in desensitizing toothpastes. Peacock et al have shown that a potassium ion concentration above 8 mM to 16 mM (0.08% to 0.16% as potassium nitrate) around the axons is needed to sustain nerve depolarization.13 While it is yet to be actually proven that the application of a toothpaste containing 5% potassium nitrate can raise the potassium concentration around the intradental nerves to the required minimal concentration, such toothpastes have been clinically proven in most published studies to alleviate tooth hypersensitivity.14-18
Based on its solubility and lack of reactivity, there is no reason to believe that any part of potassium nitrate’s mode of action is through tubule occlusion.18-20 A downside of potassium nitrate’s solubility is its transient nature, which allows it to be effective only as long as the concentration of potassium ions remains sufficiently high.18,21
Tubule occlusion represents a potentially more effective, longer-term treatment for hypersensitivity. Professional treatments, which are generally applied once to the teeth, largely depend on this mode of action to provide longer-term relief. In developing a professional treatment, the challenge is to find materials that, after a single application, remain in place for extended periods.
Toothpastes can be used twice daily for weeks, months, or even years. Such products provide a useful vehicle for desensitizing teeth and can ensure that the relief from hypersensitivity is maintained. Fluoride toothpastes are now marketed that deliver calcium and phosphate ions to the surface of the teeth. In vitro and in vivo studies have shown that these products deposit ACP on tooth enamel, filling in surface defects and restoring tooth gloss.22-24 ACP is known to transform into apatitic mineral similar to that of natural tooth enamel in the presence of moisture.25,26 Some clinical studies have shown the potential of these products to relieve hypersensitivity.27,28
Recently, a fluoride toothpaste delivering calcium and phosphate ions was developed specifically to desensitize teeth. A double-blind well-controlled clinical study has shown this product to reduce hypersensitivity more effectively than a conventional fluoride toothpaste.28 It appears likely that the mechanism of action of this and other fluoride toothpastes that deliver calcium and phosphate ions is the occlusion of tubules. The purpose of this study was to establish the mechanism of action for the desensitizing fluoride toothpaste.
Materials and Methodology
A series of experiments was performed to establish the mechanism of action of the desensitizing toothpaste.
The products evaluated in these in vitro studies were:
- Arm & Hammer® Enamel Care® Sensitive (AHECS) toothpaste (Church and Dwight Co, Inc, Princeton, NJ), a single-phase fluoride toothpaste (1100-ppm F) containing 6% calcium sulfate, 1% dipotassium phosphate, and baking soda suspended in a nonaqueous fluid. This product has been demonstrated to significantly reduce dental hypersensitivity in a well-controlled double-blind clinical study.
- A control toothpaste containing the same ingredients as AHECS, except without the calcium sulfate and dipotassium phosphate (C-1).
- A desensitizing fluoride toothpaste for home use, Sensodyne® (S) toothpaste (GlaxoSmithKline, London, United Kingdom), using 5% potassium nitrate to depolarize the nerves and stop neural transmission.
- The isolated sealants (A) from AHECS, which, when diluted, deliver a combination of calcium sulfate, dipotassium phosphate, and sodium fluoride at the same concentration as delivered by AHECS, together with a smaller quantity of baking soda to buffer the pH to that of AHECS.
- A 1.25% solution of potassium nitrate (KN).
- An insolubles-free test toothpaste, similar to AHECS, but without the insoluble silica thickener and titanium dioxide ingredients (IF).
- An insolubles-free control, similar to the insolubles-free test toothpaste but without the calcium sulfate and dipotassium phosphate ingredients (C2).
Four sets of approximately 300-µ to 500-µ thick coronal dentin discs were cut from intact human molars using a Isomet® saw (BUEHLER, Ltd, Lake Bluff, IL). Any with enamel areas toward the center or with pulp cavity horns were rejected. The disks were honed to about 50-nm smoothness by hand-rubbing with water on CarbiMet (BUEHLER, Ltd) discs with, successively, 600, 800, and 1200 mesh silicon carbide grit, followed by polishing with a 50-nm diamond suspension on a polishing cloth. Then, they were etched for 1 minute with 5% citric acid to open the tubules.
The dentin surfaces were then examined, using optical interference profilometry (OIP) on a MultiScan 5000 (Zygo Corp, Middlefield, CT), with MetroPro™ software (Zygo Corp), using a high-power (20x) objective with the magnification set to 0.5 and using a 560-nm F1 filter. Profiles of the surface were captured. Surface roughness expressed as root mean square (RMS) or arithmetical average was determined using complete scans, though occasional clipping was performed to remove obvious anomalies.
The dentin discs were then mounted in Pashley cells and perfused with dye-colored pH-7.4 phosphate-buffered saline under a static head of about 75 cm of water (0.07 atm) and the flow rate of liquid through the dentin was measured in µL/min. After several determinations of flow rate, the dentin was ready for treatment with the test product.
Treatments of the dentin with the test products were performed without removing the discs from the Pashley cell. Freshly prepared slurries containing one-part toothpaste to three-parts water were applied to the discs multiple times for 5-minute intervals, at approximately 50 g of applied force, using an Interplak® electric toothbrush (CONAIR®, Stamford, CT) with all but one set of tufts removed. The dentin was rinsed between brushings, using distilled water, and fresh toothpaste slurry was used for each application. In the case of the KN solution, a 1.25% solution (corresponding to a 1:3 dilution of a product containing 5% active KN desensitizer) was directly applied to the dentin surface, which was brushed in the usual fashion. For the isolated sealants, an aqueous slurry containing 1.5% calcium sulfate, 0.25% dipotassium phosphate, and 275-ppm fluoride (corresponding to the amounts delivered by the 1:3 AHECS slurry), as well as 0.75% baking soda (to buffer the composition to the same pH as the AHECS slurry), was directly applied, and the dentin surface was brushed.
At various periods and after all treatments were completed, the dentin discs were removed from the Pashley cells, and the dentin surfaces were re-examined using OIP. Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analyses were also performed after completion of the treatments. The SEM and EDX results were compared with SEM and EDX analyses of untreated dentin. At the completion of the final treatment, the dentin disc was broken and SEMs of cross sections were obtained. The SEM and EDX equipment was a JEOL 6400F (JEOL, Ltd., Tokyo, Japan), with INCAEnergy dispersive analyzer (Oxford Instruments, Tubney Woods, UK) and a beam energy set to 5 kV.
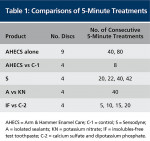
After completion of the testing with AHECS, three of the nine discs used were returned to the Pashley cell and treated for 1 minute with an acidic cola drink. The dentin permeability was then redetermined. Table 1 summarizes the comparisons that were performed.
Results
Complete Sensitive Toothpaste (AHECS)
Figure 1 exemplifies a surface profile of a pretreated and posttreated (80 treatments) dentin surface. The OIP produces a contour profile of the surface being examined, using variations in color to depict surface contours. The many black areas seen before treatment (Figure 1a) indicate locations where the tubule entrances are present. Almost none are visible after treatment (Figure 1b), and an overall surface layer of mineral is indicated by the large variations in color seen over the scanned surface. One of the surface parameters, which can be measured by OIP, is surface roughness. Before treatment, the average RMS roughness of the relatively smooth dentin was 0.4. A total of 80 treatments of the dentin with AHECS resulted in increase in RMS to 1.9 because of mineral deposition.
Figure 2 provides SEMs showing open tubules in untreated dentin and shows tubule occlusion of AHECS-treated dentin. The treated surface appears relatively uniform without the appearance of individual-deposited particles (Figure 2b). Figure 3 provides SEM images of cross sections of untreated and AHECS-treated dentin. Careful examination of the untreated dentin surface (Figure 3a) shows no mineral layer and the clear tubules. In contrast, a thin layer of mineral is visible on the AHECS-treated surface (Figure 3b). This layer of mineral occludes most of the tubules.
EDX analysis of the surface indicates the presence of large amounts of calcium, some sulfur and some phosphorous. Based on this evidence, the occluding material is likely to be some form of calcium phosphate with some residual calcium sulfate also present. In addition, significant levels of titanium, presumably as titanium dioxide, and a small amount of silicon, presumably in the form of silica, can be seen. Both silica and titanium dioxide are present in AHECS.
Table 2 summarizes the reductions in flow rate through all nine treated disks after 40 and 80 treatments with AHECS. After completion of the 80 treatments and performance of surface analyses, three of the dentin disks were returned to the Pashley cell and challenged with an acidic cola beverage for 1 minute. This produced an average 75.4% increase in dentin permeability, demonstrating the acid-soluble nature of the dentin permeability-reducing deposit.
Thus, these results showed that AHECS deposits onto the surface of the dentin a layer of calcium phosphate mineral, which is presumed to be ACP. In the presence of moisture, this rapidly transforms into calcium hydroxyapatite (HAP), forming a coherent mineral layer, which occludes the dentinal tubules and reduces dentin permeability. As noted above, some titanium dioxide, silicon dioxide, and calcium sulfate are also present in this layer.
Comparison of the Complete Sensitive Toothpaste (AHECS) with Control (C-1)
SEMs of the C-1-treated dentin are shown in Figure 4. The presence of small particles, partially covering the surface and occluding some of the tubules, can be seen (Figure 4a). The particulate nature of the deposit in this SEM is readily seen in the close-up image (Figure 4b). It contrasts with the coherent deposit seen in Figure 2 and Figure 3, resulting from treatment of dentin with AHECS.
EDX analysis of the C-1-treated surface indicates the presence of significant quantities of titanium dioxide, which is present in the control formulation. Interestingly, no silica can be detected. There are also calcium and phosphate peaks, which are from calcium phosphate mineral, which is in the underlying dentin. This calcium phosphate in the dentin is in the form of calcium-deficient HAP as shown by a calcium-to-phosphorous ratio of about 1.5.
Table 3 compares the percent flow reduction obtained for the AHECS and C-1 toothpastes vs number of treatments. Both products reduced dentin permeability, and there was no significant difference in permeability reduction between the two products.
These results show that, while only AHECS deposits a coherent layer of calcium phosphate mineral onto the surface of the dentin, C-1 deposits many insoluble particles onto the dentin and into the tubules. While the two deposits appear very different, both effect a reduction in the permeability through dentin when evaluated using the Pashley cell.
Potassium Nitrate-Containing Toothpaste (S)
Figure 5 provides an SEM visualization of a dentin surface that had been treated 42 times with S, the desensitizing toothpaste containing potassium nitrate. A particulate residue can be seen on the dentin (Figure 5a). The close-up shows that the particulate residue is present in the entrance to the tubules (Figure 5b).
EDX analysis of the surface indicates the presence of large amounts of silicon dioxide, which almost completely covers the dentin surface in the area evaluated. Because of the coverage, carbon, calcium, and phosphorous peaks from dentin are not seen. It seems that silica coverage is more extensive than apparent in the SEM images.
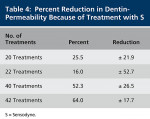
Table 4 shows that S produces highly variable reductions in dentin flow rate (high standard deviations). After 42 sequential treatments, the average reduction in flow rate was 64.15%, which was similar to that obtained after 40 treatments of dentin with AHECS (57.4%).
Comparison of Isolated Sealants (A) with Potassium Nitrate (KN)
As noted previously, OIP visualization of the untreated dentin surface shows the dentinal tubules as uneven oval black spots. After 40 treatments with A, the isolated actives, OIP indicates that the tubules are blocked with an uneven layer of mineral. In contrast, after 40 treatments of dentin with potassium nitrate solution, the open tubules can be clearly seen as black areas and no deposits of mineral are present.
Figure 6 provides an SEM of dentin treated with A. A highly mineralized surface deposit is seen on the isolated sealants-treated dentin, and the tubules are blocked (Figure 6a). Also shown is an SEM of the KN-treated dentin (Figure 6b). No mineralization is visible, and the open tubules are clearly visible.
EDX analysis of the dentin surfaces treated with A show that it is covered with a mineral-containing calcium and phosphate in the atomic ratio of about 1.7 to 1. This is consistent with the presence of HAP or perhaps amorphous calcium phosphate (ACP), which theoretically has a calcium-to-phosphate ratio of 1.66. Only a very small carbon peak is detectable, indicating that that much of the protein (collagen) surface is covered by this mineral.
The EDX of the KN-treated disc shows bare dentin. The high carbon peak is indicative of protein, while the calcium and phosphate peaks, which are in the ratio of 1.4 to 1, would indicate the presence of “depleted” calcium hydroxyapatite, typical of the mineral remaining in the surface of etched dentin.
After 40 treatments with A, the average reduction in flow rate through the dentin discs was 39.5% compared to 10.6% for the potassium nitrate solution (Table 5). The difference in flow reduction for the two sets of treatments was significant (P < .02).
This demonstrates that the occluding ingredients in AHECS are capable of producing a calcium phosphate mineral on dentin, which reduces dentin permeability. In contrast, potassium nitrate, the active ingredient in S, as expected, does not occlude tubules and does not operate by reducing dentin permeability.
Comparison of Insolubles-Free Test Toothpaste (IF) with Insolubles-Free Control (C-2)
SEMs of the discs treated with IF show mineral deposits on the dentin. In some areas there is complete occlusion of the tubules (Figure 7a); in others the occlusion is significantly less. However, none of the dentin treated with C-2 exhibited visible deposits (Figure 7b), and the tubules remain completely open.
EDX analysis of the dentin surfaces treated with the IF is consistent with the presence of HAP or ACP on the dentin surface. However, EDX analysis of the C-2-treated dentin is typical of demineralized dentin, ie, a strong carbon peak from collagen and weaker calcium and phosphate peaks from calcium-deficient apatite mineral.
The flows through in the dentin after five, 10, 15, and 20 5-minute treatments with IF and C-2 are summarized in Table 6. It can be seen that C-2 treatment has no significant effect on fluid flow through dentin. Statistical t-test comparisons show that after 15 and 20 treatments, the reductions in flow rates through the dentin were significantly greater for IF than C-2 (P = .04 and P = .027, respectively).
This comparison shows that when the calcium and phosphate ingredients are incorporated into a fluoride toothpaste similar to AHECS but free of insoluble ingredients such as titanium dioxide and silica, they maintain their ability to block tubules and inhibit tubule flow. The control calcium- and phosphate-free product with no insolubles leaves no residue, and the dentin is essentially unchanged by the treatments.
Discussion
The initial evaluation of AHECS confirms its ability to deposit mineral on dentin, occlude dentinal tubules, and reduce dentin permeability in the Pashley cell. The material initially formed on dentin is likely to be ACP, which then transforms relatively rapidly in the presence of moisture into apatitic calcium phosphate as a coherent mineral layer. However, some titanium dioxide, silica, and calcium sulfate residues from the toothpaste formulation can be detected in this mineral layer on the dentin. That the dentin permeability-reducing substance is not resistant to acidic cola exposure provides further evidence that the substance blocking the tubules is an acid-soluble calcium phosphate, such as HAP, rather than titanium dioxide, silica, or calcium sulfate.
The results also show that the calcium- and phosphate-free control (C-1) also deposits particles on the surface primarily in the form of titanium dioxide and the commercial desensitizing toothpaste (S) containing potassium nitrate, deposits particles of silica. The dentin permeability test indicates that these deposits also reduce dentin permeability in the Pashley cell.
The presence of residue on dentin after treatment with conventional toothpastes has also been reported by others. Thus, Pashley et al showed that desensitizing toothpastes with the actives removed, as well as conventional (nondesensitizing) dentifrices, often produced as large a reduction in dentin permeability as active desensitizing tooth-pastes.29 These workers even proposed that at least part of the desensitizing activity of desensitizing toothpastes is because of the presence of the supposedly inactive insoluble ingredients. Gillam et al similarly found that much if not all of the tubule occluding activity of toothpastes was because of the insoluble abrasives rather than active desensitizing ingredients.2
Conversely, West et al found that insoluble abrasives generally have the potential to abrade dentin and open the tubules. However, they did find that some abrasive particles, especially synthetic silicas, have an attraction to the dentin, resulting in tubule occlusion.30,31 Addy et al reported than many insoluble abrasives were deposited on dentin and occluded tubules.32 However, most of these could be readily washed off. In their studies, only fumed silica was sufficiently bound to the surface to remain after rinsing. Absi et al confirmed the ready removal of most insoluble abrasives and found that some silicas are resistant even to removal by dietary acids.33
While insoluble abrasives, such as silica abrasives, are not generally considered sufficiently effective as the primary agent for the treatment of hypersensitivity, it has been proposed as a possible reason why placebo dentifrices often perform better than expected in clinical trials. In general, however, the relief provided by such insoluble ingredients is probably insufficient as a worthwhile treatment for hypersensitivity in most cases.
Several possible reasons may explain why the results of the dentin permeability test using the Pashley cell are not reflective of the actual hypersensitivity-reducing performance of toothpastes. First, the discs used in the standard dentin permeability test are cut from coronal dentin. Because of differences between coronal and root dentin, for example, in the number of exposed tubules per unit area and in the geometry of the tubules and their size, there may be significant differences in the way the two types of dentin trap insoluble particles, which might affect fluid flow.
Secondly, during the dentin permeability test, the dentin is held in the Pashley cell in the horizontal position, while the treatments are applied by brushing onto the dentin surface. Gravity acting on the insoluble particles from the dentifrice, coupled with the brushing action, may help force them deeper into the tubules, and, as a result, they may significantly reduce dentin permeability to a greater degree than in vivo. In vivo, toothpaste is applied to exposed dentin, which is oriented approximately vertically.
Markowitz et al proposed what seems like the most likely reason for failure of the dentin permeability test to differentiate between the test toothpaste and controls.34 They suggest that the permeability test may not always be sensitive enough to detect changes in dentinal flow produced by pain-causing stimuli (eg, evaporation flow) because of an air blast. Thus, Pashley et al found that, while a smear layer on dentin can reduce hydrostatically induced fluid flow in dentin by 90%, air blast-induced fluid flow was reduced by only 50%.35 It seems that an air blast onto closely packed particles mixed with water can induce evaporation of some moisture and the surrounding fluid can be induced to flow through capillary action. This type of fluid flow cannot be detected in the dentin permeability test because it is too small and perhaps because hydrodynamic pressure causes the particles to pack closer together.
In this study, insoluble particles from C-1 as well as from the potassium nitrate-containing toothpaste occluded tubules and reduced fluid flow in the dentin permeability test. However, no evidence suggests these particles bond with the dentin or form a coherent layer across the tubules and thus prevent evaporative flow from an air blast. In fact, SEMs of the treated dentin surface show the individual particles. Conversely, AHECS and those treatments that contained sources of calcium and phosphate produced a nonparticulate, coherent layer of mineral that should resist evaporative flow.
This study also showed that, in the absence of insoluble ingredients, the isolated sealants are effective in forming calcium phosphate mineral on dentin and in reducing dentin permeability. In contrast, potassium nitrate, which is considered to operate by depolarizing the nerves rather than by occluding tubules, leaves no residue on dentin and does not reduce dentin permeability.
Conclusions
The calcium and phosphate ingredients in this desensitizing toothpaste are responsible for its effectiveness in the relief of hypersensitivity. When applied to the teeth, ACP is formed as a layer on the dentin. ACP rapidly transforms into apatitic mineral, which seals the tubes and prevents stimuli from reaching the intradental nerves. In contrast to other toothpastes that leave particulate residues on dentin, the therapeutic effectiveness of this toothpaste is strongly related to the deposition of a mineral that is coherent and strongly bonded to the dentin surface.
Disclosure
This research was supported by Church & Dwight Co, Inc. Mr. Charig and Ms. Chapin were employees of Church and Dwight, and Ms. Major and Dr. Thong are current employees. Dr. Thong is a shareholder in Church and Dwight. Mr. Winston is a former Church and Dwight employee. He is a shareholder and serves as a consultant for the company.
References
1. Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity–an enigma? A review of terminology, epidemiology, mechanisms, aetiology and management. Brit Dent J. 1999;187(11):606-611.
2. Gillam, DG, Mordan, NJ, Newman HN. The Dentin Disc surface: a plausible model for dentin physiology and dentin sensitivity evaluation. Adv Dent Res. 1997;11(4):487-501.
3. Addy M. Dentin hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367-375.
4. Kielbassa AM. Dentine hypersensitivity: simple steps for everyday diagnosis and measurement. Int Dent J. 2002;52: 394-396.
5. Draper C. Dentin hypersensitivity: understanding the causes and treatment strategies. Contemporary Oral Hygiene. 2008;6(4):11-19.
6. Walters PA. Dentinal hypersensitivity: a review. J Contemp Dent Pract. 2005;6(2): 107-117.
7. Brannstrom M. A hydrodynamic mechanism in the transmission of pain producing stimuli through dentin. In: Anderson DJ, ed. Sensory Mechanism in Dentine. Oxford, UK: Pergammon Press; 1963:73-79.
8. Sena FJ. Dentinal permeability in assessing therapeutic agents. Dent Clin North Am. 1990;34(3):475-490.
9. Kim S. Hypersensitive teeth: desensitization of pulpal sensory nerves. J Endod. 1986;12(10):482-485.
10. Absi EG, Addy M, Adams D. Dentine hypersensitivity. The development and evaluation of a replica technique to study sensitive and non-sensitive dentine. J Clin Periodontol. 1989;16(3):190-195.
11. Vongsavan N, Matthews B. The permeability of cat dentine in vivo and in vitro. Arch Oral Biol. 1991;36(9):641-646.
12. Vongsavan N, Matthews B. Fluid flow through cat dentine in vivo. Arch Oral Biol. 1992;37(3):175-185.
13. Peacock JM, Orchardson R. Effects of potassium ions on action potential conduction in A- and C-fibers of rat spinal nerves. J Dent Res 1995;74(2):634-641.
14. Silverman G, Berman E, Hanna CB, et al. Assessing the efficacy of three dentifrices in the treatment of dentinal hypersensitivity. J Am Dent Assoc. 1996;127(2):191-201.
15. Tarbet WJ, Buckner A, Stark MM, et al. The pulpal effects of brushing with 5 percent potassium nitrate paste used for desensitization. Oral Surg Oral Med Oral Pathol. 1981;51(6):600-602.
16. Poulsen S, Errboe M, Hovgaard O, et al. Potassium nitrate for dentine hypersensitivity. The Cochrane Database Syst Rev. 2006;19(3):CD001476.
17. Orchardson R, Gillam DG. The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain. 2000;14(1):9-19.
18. Wara-aswapati N, Krongnawakul D, Jiraviboon D, et al. The effect of a new toothpaste containing potassium nitrate and triclosan on gingival health, plaque formation and dentine hypersensitivity. J Clin Periodontol. 2005;32(1):53-58.
19. Poulsen S, Errboe M, Hovgaard O, et al. Potassium nitrate toothpaste for dentine hypersensitivity (review). The Cochrane Collaboration. Hoboken, NJ: John Wiley & Sons, Inc.; 2004:1-11.
20. Knight NN, Lie T, Clark SM, et al. Hypersensitive dentin: testing of procedures for mechanical and chemical obliteration of dentinal tubuli. J Periodontol. 1993;64(5):366-373.
21. West NX, Addy M, Jackson RJ, et al. Dentine hypersensitivity and the placebo response. A comparison of the effect of strontium acetate, potassium nitrate and fluoride toothpastes. J Clin Periodontol. 1997;24(4): 209-215.
22. Charig A, Winston A, Flickinger M. Enamel mineralization by calcium-containing bicarbonate toothpastes: assessment by various techniques. Compend Contin Educ Dent. 2004;25(9 suppl):14-24.
23. Litkowski LJ, Quinlan KB, Ross DR, et al. Intraoral evaluation of mineralization of cosmetic defects by a toothpaste containing calcium, fluoride and sodium bicarbonate. Compend Contin Educ Dent. 2004;25(9 suppl 1):25-31.
24. Muñoz CA, Stephens JA, Proskin HM, et al. Clinical efficacy evaluation of a calcium, phosphate, and sodium bicarbonate on surface-enamel smoothness and gloss. Compend Contin Educ Dent. 2004;25(9 suppl 1)25: 39-39.
25. van Kemenade MJJM, de Bruyn PL. A kinetic study of precipitation from supersaturated calcium phosphate solutions. J Colloid Interface Sci. 1987;118(2): 564-585.
26. Tung MS, Eichmiller FC. Dental applications of amorphous calcium phosphates. J Clin Dent. 1999;10(1 spec no):1-6.
27. Kaufman HW, Wolff MS, Winston AE, et al. Clinical evaluation of the effect of a remineralizing toothpaste on dentinal sensitivity. J Clin Dent. 1999;10(1 spec no):50-54.
28. Ghassemi A, Hooper W, Winston A, et al. Effectiveness of a baking soda-toothpaste, delivering calcium and phosphate, in reducing dentinal hypersensitivity. J Clin Dent. In press.
29. Pashley DH, O’Meara JA, Kepler EE, et al. Dentin permeability effects of desensitizing dentifrices in vitro. J Periodontol. 1984;55(9):522-525.
30. West N, Addy M, Hughes J. Dentine hypersensitivity: the effects of brushing desensitizing toothpastes, their solid and liquid phases, and detergents on dentine and acrylic: studies in vitro. J Oral Rehabil. 1998;25(12): 885-895.
31. West NX, Hughes JA, Addy M. Dentine hypersensitivity: the effects of brushing toothpaste on etched and unetched dentine in vitro. J Oral Rehabil. 2002;29(2):167-174.
32. Addy M, Mostafa P. Dentine hypersensitivity. II. Effects produced by the uptake in vitro of toothpastes onto dentine. J Oral Rehabil. 1989;16(1): 35-48.
33. Absi EG, Addy M, Adams D. Dentine hypersensitivity: uptake of toothpastes onto dentine and effects of brushing washing and with dietary acid–SEM in vitro study. J Oral Rehabil. 1995;22(3): 175-182.
34. Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008;35(4): 300-315.
35. Pashley DH, Matthews WG, Zhang Y, et al. Fluid shifts across human dentine in vitro in response to hydrodynamic stimuli. Arch Oral Biol. 1996;41(11):1065-1072.
About the Authors
Andrew J. Charig, BS
Senior Chemist (retired)
Church & Dwight Co, Inc
Princeton, New Jersey
Charity A. Chapin, BS
Technician (formerly)
Church & Dwight Co, Inc
Princeton, New Jersey
Elizabeth E. Major, BS
Consultant
Church & Dwight Co, Inc
Princeton, New Jersey
Stephen Thong, PhD
Director
Global Oral Care R&D for Oral Care Products
Church & Dwight Co, Inc
Princeton, New Jersey
Anthony E. Winston, BSc
President
R&D for Hire
East Brunswick, New Jersey