Strength of Evidence Relating Periodontal Disease and Atherosclerotic Disease
Kaumudi Joshipura, BDS, MS, ScD; Juan Carlos Zevallos, MD; Christine Seel Ritchie, MD, MSPH
Abstract
This review assesses the strength of evidence relating periodontal disease and atherosclerotic disease (ischemic heart disease, peripheral arterial disease, and ischemic stroke). Periodontal disease and atherosclerotic disease may be linked causally, or their relationship could be explained, wholly or partially, by common risk factors. Many potential pathways for the relationship have been postulated. This article focuses on evaluating the overall body of evidence, according to the following standard causal inference criteria: strength of association, dose-response relationship, time sequence, consistency, specificity, biologic plausibility, and independence from confounding. Each criterion is reviewed and evaluated against the existing literature. In summary, the overall strength of evidence for causal criteria for the relation between periodontal disease and atherosclerotic disease is as follows: The magnitude and consistency of the association is stronger for ischemic stroke (and is low for ischemic heart disease), some evidence for dose response exists, time sequence has been established with more evidence for stroke, and there is definitely biologic plausibility for all these associations. Independence from confounding is also stronger for ischemic stroke and peripheral arterial disease. Specificity is not established for any of these associations, as there are multiple risk factors for atherosclerotic disease; however, specificity is not considered an important criterion for causality. Because the underlying pathogenesis of atherosclerosis is common across the diseases, it is likely that if additional studies show consistent associations, periodontal disease may be an important independent causal risk factor for atherosclerotic disease, especially for ischemic stroke.
Cardiovascular disease (CVD) encompasses atherosclerotic disease (including ischemic heart disease [IHD], peripheral arterial disease [PAD], and ischemic stroke); hemorrhagic stroke; congestive heart failure; hypertension; rheumatic heart disease; and congenital heart defects. This article reviews the evidence relating periodontal disease with CVD arising from atherosclerosis (ie, IHD, PAD, and ischemic stroke). The term IHD is used interchangeably with coronary heart disease (CHD).
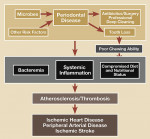
Inflammation is recognized as playing a key role in the pathogenesis of atherosclerosis. Inflammatory cells and cytokines are important not only in the initiation of plaque formation in the blood vessel wall but also in the maintenance and rupture of the plaque and subsequent thrombotic complications. Triggers of inflammation include smoking, diabetes mellitus, and obesity.1,2 These factors (potential confounders) have been related to both periodontal disease and CVD in several studies (Figure 1) and could lead to apparent associations even if there were no causal relationship.
Several possible biologic pathways for the relationship between periodontal disease and atherosclerotic disease have been postulated as summarized in Figure 2. Periodontal disease may increase systemic levels of inflammatory mediators and thus potentially contribute to the inflammation-associated atherosclerotic process.3 Patients with periodontal disease manifest increased local expression of host inflammatory mediators, such as prostaglandins, interleukin-1, and tumor necrosis factor-a. Periodontal pathogens also may disseminate into the systemic circulation and localize in atheromas.4 Deshpande et al5 demonstrated that Porphyromonas gingivalis can invade aortic and heart endothelial cells via fimbriae. Alternatively, individuals with periodontal disease and atherosclerotic disease may share common behaviors or have common host responses to inflammation (implying a noncausal relationship). For example, those most likely to require dental care may be most likely to have behaviors that may increase the risk of atherosclerosis (eg, smoking, decreased physical activity). Alternatively, sequelae of periodontal disease (ie, tooth loss) may lead to dietary changes, such as decreased intake of fruits and vegetables/dietary fiber that subsequently could increase the risk for atherosclerotic and other diseases. Also, it is possible that those who are genetically susceptible to systemic inflammation may demonstrate increased oral inflammation in the form of gingivitis or periodontal disease, as well as increased risk of atherosclerotic disease. Because of these complex relationships, it is difficult to assess whether oral diseases actually contribute to increased risk of atherosclerotic disease (as a causal relationship) or if oral and atherosclerotic diseases share common risk factors. This article attempts to review the current evidence to understand the strength of the evidence and to provide some insight into possible causality of the relationship.
Causal Inference Criteria
It seems likely that there could be a combination of common risk factors (shown in the noncausal pathways in Figure 1), which could explain some of the association, as well as some causal pathways. To assess the possible existence of a causal component independent of common risk factors, this article reviews the major prospective studies in the context of the criteria for causality proposed by Hill.6 Some of these criteria have been challenged or have evolved. However, these basic criteria, still considered a standard approach for assessing causality,6,7 are defined individually and applied to the pertinent literature as in the authors’ previous publication.8 These criteria include strength of association, dose-response relationship, time sequence, consistency, specificity, and biologic plausibility. For the purposes of this article, the authors combined coherence and plausibility into the criterion of biologic plausibility as the differences between the two are very subtle.7 Also, the criterion of experiment was not assessed because there is no direct evidence to date from clinical trials for the clinical outcomes, and it is not possible to allocate people randomly to periodontal disease (only to periodontal treatment). Only one small pilot randomized control trial assessed the impact of periodontal treatment9 on adverse events, such as cardiovascular and noncardiovascular, and it was not possible to gauge the impact on cardiovascular outcomes. Hence, this article does not discuss this study. Lastly, the criterion of analogy has been excluded because as Rothman and Greenland argued, “scientists...can find analogies everywhere” and “the absence of such analogies only reflects lack of imagination or experience.”10
Some epidemiologists have proposed alternative criteria for causality. Rothman defined a causal mechanism as a set of factors that are jointly sufficient to induce an outcome event and minimally sufficient; that is, under the omission of just one factor, the outcome would change.11 This definition highlights the potential complexity of causality but provides less structure for evaluating the effect of one condition on another outcome. For this article as in their earlier review,8 the authors evaluated the relationship between periodontal disease and CVD in the context of Hill’s criteria, recognizing the inherent limitation in any set of criteria used to assess causality.
Many studies have evaluated the association between periodontal disease and CVD, including IHD, PAD, and ischemic stroke. Although Mattila and colleagues’ early work12 deserves credit for stimulating interest in this area of research and there are several subsequent case-control and cross-sectional studies with varying degrees of methodologic rigor, the authors have focused on only the longitudinal studies for this review (Table 1).
Strength of Association
For this criterion, Hill argued that a strong statistical association is more likely to have a causal component than a modest association because large associations are less likely to be caused by small biases, randomness, or confounding. However, the absence of a strong association does not rule out a causal effect.
The first prospective study was conducted by DeStefano and colleagues.13 This report was based on a 14-year follow-up study of the First National Health and Nutrition Examination Survey (NHANES I) participants and demonstrated a significant relative risk of 1.25 (25% increased risk) for IHD, comparing those with periodontal disease to those without. The study by Hujoel et al14 re-analyzed the NHANES I data set (used in DeStefano and colleagues’ study13) with longer follow-up, controlling more rigorously for confounding factors; they found no relationship. Joshipura et al published a study15 with more than 40,000 men showing no overall association between periodontal disease and IHD. However, periodontal disease was significantly associated with increased IHD risk among participants who had very few teeth. Beck and colleagues16 showed a significant increase in IHD risk among those with periodontal disease. Four studies assessed fatal IHD: The studies by Morrison et al17 and Tuominen et al18 did not show significant associations. Saremi et al19 showed a marginal association between severe periodontal disease and fatal IHD, which was significant only when combined with mortality from diabetic nephropathy into cardio-renal mortality among Pima Indians who had type 2 diabetes. A recent study by Dietrich et al20 found a significant association among the younger subgroup (< 60 years) between periodontal disease and IHD among US veterans but not in those older than 60 years of age. Two studies evaluated secondary outcomes of IHD in those who already had one myocardial infarction (Table 1). The study by Mattila and colleagues21 showed a significant relationship, while research by Hujoel and colleagues22 did not. Only two studies have considered the relationship between PAD and periodontal disease,23,24 and both showed significantly elevated risk of PAD among participants with periodontal disease (Table 2). For stroke, four of the six studies consistently showed significantly elevated relative risks (Table 2). The significant relative risks ranged from 1.2 to 2.1 for IHD, 1.4 to 2.3 for PAD, and 1.3 to 2.8 for stroke. Of the two remaining studies, one showed no association25 and one demonstrated an elevated but insignificant relative risk.17
Tooth Loss and CVD
Studies that focused on the relationship between tooth loss and CVD (Table 3) also have been considered as part of the supporting evidence because tooth loss partially reflects antecedent periodontal disease. For tooth loss and IHD, three studies did not show any overall relationship,18,20,25 whereas a significant relationship was seen in three cohorts.17,26 In Joshipura and colleagues’ 1996 report,15 a significant relationship was observed for the combination of tooth loss and periodontal disease; the relative risk for tooth loss was elevated but not significant. The subsequent report by Hung et al26 with longer follow-up found significant associations between tooth loss and IHD in the same cohort of male professionals as well as in an additional group of women. The relationship between PAD and recent tooth loss showed a stronger association than tooth loss that occurred in the distant past. That is, tooth loss in the past 6 years was associated with elevated risk for PAD, but the baseline number of teeth was not significantly associated with PAD24 (Table 3). For stroke, only one study showed a significant association for tooth loss.27
When comparing periodontal disease and tooth loss, it seems that, overall, periodontal disease and tooth loss demonstrate similar relationships with IHD and stroke. Among health professionals, recent tooth loss followed the same pattern as periodontal disease (significant for PAD and stroke27 but not for IHD15,26). This may be expected because if people lose teeth in their 40s and 50s, it is likely a result of periodontal disease.
Morrison et al17 showed a significant association between edentulousness and IHD but not with stroke within a population of Canadians. Dietrich et al20 showed a strong significant association between tooth loss and fatal IHD when they compared edentulous individuals with people exhibiting no periodontal bone loss among men 60 years and older; no significant association was seen with total CHD in the whole population. The five other studies reviewed showed no significant association. In summary, according to the strength-of-association criteria, the link between periodontal disease and CVD appears stronger for both stroke and PAD than for IHD, as also is suggested from the meta-analyses. In meta-analyses that included many of these studies, pooled results demonstrated a weak and inconsistently significant association between periodontal disease and IHD and a stronger but still inconsistently significant association between periodontal disease and stroke. The validity of pooling these results in meta-analyses is negatively influenced by limitations in the original studies, including variations in measurements of both periodontal disease and IHD outcomes and in the control of confounders.
Dose-Response Relationship
To fulfill this criterion, the outcome should increase with increasing dose of exposure. A dose-response relationship is not always found in causal relationships, in which case a more complex explanation of the relationship may be required.7
Very few studies have evaluated dose response. Beck and colleagues16 and Geerts and colleagues (case-control study)28 assessed dose response, relating increasing levels of periodontal disease with IHD risk. Both studies found a significant dose-response relationship. Recently, Dietrich et al20 found a dose-dependent association between chronic periodontitis and incidence of IHD in the Veteran Affairs Normative Aging and Dental Longitudinal Study. The risk of developing IHD increased with higher cumulative probing depths (hazard ratio = 1.10; 95% confidence interval 1.05 to 1.17; per 10-mm increment; P < .001) among men younger than 60 years of age. However, there was no such linear association among those older than 60 years.20 A study by Beck et al16 assessed dose-response relationship for stroke but did not find any linear association.
Time Sequence
The potential causal factor must precede the outcome that it is assumed to affect for the time-sequence criteria to be met. Other authors define this criterion as “lack of temporal ambiguity.”29 This is best ascertained in longitudinal studies and ideally in randomized controlled trials when it is practicable and ethical to allocate the postulated causal factor randomly.
In several studies, the exposure clearly preceded the outcome. From the longitudinal studies to date, three of the 11 IHD studies, both PAD studies, and four of the six stroke studies showed a temporal relationship (Table 2). Because periodontal disease precedes the outcome (atherosclerotic disease) in the longitudinal studies, these studies provide much better support for causal inference concerning the relationship between periodontal disease and CVD compared with case-control and cross-sectional studies. However, given that both periodontal disease and atherosclerotic disease are chronic conditions, it is challenging to conclude, even from longitudinal studies, whether the periodontal disease truly preceded the early stages of atherosclerosis. Conversely, it seems unlikely that atherosclerotic disease could cause periodontal disease. Hence, there does seem to be evidence, strongest for stroke but also for IHD and PAD, suggesting that the time-sequence criterion has been met.
Consistency
If several studies show similar results, it can be said that the relationship is consistent. Consistently finding an association with different study designs and populations reduces the likelihood that an association would be explained by a “constant” error in the design.
For IHD, many studies found insignificant results, and overall, the results were not consistent (Table 1). Therefore, more IHD studies are needed to corroborate the relationship. The relationship is most consistent for stroke, in which four16,27,30,31 of the six studies found an elevated relationship (Table 2). Both studies on PAD23,24 show consistent results, but this needs to be replicated in more longitudinal studies.
The possible explanations for the inconsistency for IHD could include chance variation (some studies observed an association by chance). Alternatively, differences in population characteristics, limitations in the studies of exposure measures, outcome measures, or control of confounders may explain the inconsistencies. Site differences (eg, different proximities between the heart and brain as they relate to the mouth) or small differences in arterial flow between coronary, peripheral, and cerebral vasculature may explain some of the differences in associations across different CVD outcomes.
Limitation in Exposure Measures
Periodontal exposure measures vary across studies. Pocket depth, attachment loss, and bone loss are the standard population-based measures for periodontal disease; however, there is no universal definition or cutoff for periodontal disease. Therefore, the threshold is not predefined and the measures vary. In addition, the possibility exists that teeth with more severe periodontal disease were extracted. Hence, there are limitations even in the “standard” measures.
Some studies use composite measures of a total dental index; however, because caries, tooth loss, and periodontal disease are together in one index, it is difficult to distinguish what exposure truly is related to atherosclerotic disease outcomes. Tooth loss as an exposure also could be considered a surrogate marker for periodontal disease. Because caries, orthodontic needs, and trauma also could contribute to tooth loss, especially in childhood, recent tooth loss among adults better reflects periodontal disease.
Self-reported measures of periodontal disease have been criticized for providing limited information and because of the inability of participants to recognize subtle changes in periodontal status. However, if an association is observed using self-reports, it is unlikely that the bias from the measure is in the direction of observing a stronger association.32,33 Rather, random misclassification of a binary measure generally biases the estimates toward the null, hence the associations observed using self-reported data (expected to be randomly misclassified with respect to atherosclerotic disease) are likely to be attenuated compared with those using the clinical measures.32,33 The self-reported measures used in the studies reported by Joshipura’s group15,24,26,27 showed good validity against radiographic bone loss among dentists34 and other health professionals.35 Self-reports also were found to perform just as well as the clinical periodontal measures in assessing a linear relationship with age.35 Importantly, these same measures show associations with some outcomes but not with other outcomes in the same populations.
Limitations in Atherosclerotic Disease Outcome Measures
Atherosclerotic disease outcomes also are assessed differently across studies. Angina, which is a softer measure than myocardial infarction, was included in the IHD outcome in some studies, causing some misclassification. Some stroke studies focused on ischemic stroke, some combined different types of stroke (both hemorrhagic and ischemic), and some included transient ischemic attack (a transient occlusion of a cerebral vessel). In addition, outcomes in the atherosclerotic disease studies varied from fatal to nonfatal to both. The degree of verification of the atherosclerotic disease outcome also varies across studies. These limitations must be kept in mind when considering inconsistency.
Limitations in Controlling for Confounding
This is addressed in the section on confounding and could possibly explain the inconsistencies in the results.
Population Differences
Finally, there could be genuine differences between the populations studied that could lead to differences in associations if the associations exist only among subgroups, such as younger people, smokers, etc. The population differences in race, socioeconomic status (SES), or smoking status may explain some of the inconsistencies. In summary, consistency is lacking for IHD but is reasonable for stroke.
Independence from Confounding
A confounder is an extraneous factor that leads to an apparent association between the exposure and outcome that is different from the true association. This criterion was not mentioned explicitly in the Hill criteria and was introduced in the authors’ 2000 article8 because of concerns about confounding control in some studies relating oral conditions and systemic outcomes.36 One reason it was omitted earlier may be that it was subsumed under the other criteria. Given the multitude of confounders and complexity of adjusting for some of them, these authors feel it is important to emphasize this criterion separately. The association between periodontal disease and atherosclerotic disease should be independent of confounding, at least from the major known risk factors for these diseases. Unless it can be shown that the association is independent of common risk factors, causal interpretation is meaningless.
Confounders can lead to a deceptive association between two factors (Figure 1). If a study finds an association between periodontal disease and atherosclerotic disease, it may imply that periodontal disease causes atherosclerotic disease, or it could just imply that common risk factors could cause both. For example, age and smoking are confounders and both increase the risk for the periodontal exposure and atherosclerotic disease outcome. Because of confounders, a relationship could be seen between periodontal disease and ischemic stroke even if there were no causal relation. In the design of a study, one good way to control for confounding is randomization. Clinical trials are advantageous because, if randomized and sufficiently large, they are likely to be virtually free of confounding. Often randomization is not feasible or is too costly. In these instances, the optimal observational study will control for all of the important confounding variables. One is only “over-controlling” if controlling for mediators. Mediators are part of the biologic pathway and occur in time, between the exposure and the outcome, but confounders are just common risk factors. Differentiating mediators and confounders often is difficult analytically, especially in the complex biologic models of inflammation.
Hujoel has emphasized the importance and difficulty of adequately controlling for smoking.37 Danesh et al have criticized several studies for not adequately controlling for SES.38 In addition to factors that can be measured and controlled, some are hard to measure and control for, such as health awareness or health behavior. Studies focusing on relatively homogeneous populations, such as health professional groups, are able to partly control for such factors. Hujoel tabulated the degree of control of confounding by smoking dose and health awareness in various prospective studies37 and mentioned that, in the studies among health professionals, health awareness and behavior were controlled, at least partially, by using this population. These professionals knew more about health and, therefore, likely did more to prevent CVD as well as oral diseases. Among the 11 prospective studies relating periodontal disease and IHD shown in Table 1, two controlled for health awareness15,25 and eight for SES in some manner.13-15,18,20-22,25 The study by Saremi et al19 did not control for SES (partial control may have been achieved as free dental and medical care was available to all participants). Both PAD studies23,24 controlled for SES, one of which also controlled for health awareness. Among the six stroke studies in Table 2, two25,27 controlled for health awareness and all but one17 controlled for SES. Because the studies among health professionals were the only ones that had the opportunity to control for health awareness, positive associations in this population provide stronger evidence for independence from confounding. Among health professionals, positive associations were found between periodontal disease and ischemic stroke and PAD.
Specificity
Specificity is one of the criteria postulated by Hill, but many do not regard it as relevant when applied to complex redundant immunomodulatory pathways. It can be called upon as a criterion when a single putative cause produces a specific effect. This is not true for CVD or many other diseases, because multiple pathways can lead to the same outcome. Specificity provides additional support for causality, but absence of specificity (multiple causes) as in CVD does not negate a causal relationship.
Biologic Plausibility
Ideally, the observed association should be biologically explainable and should not contradict present overall scientific knowledge. After a statistical relationship is found, it needs to be determined if it is biologically plausible. Generally, if the epidemiologic associations are established, causality is more likely if a supported biologic explanation exists for it. There are many biologically plausible explanations for periodontal disease to be related with atherosclerotic disease as shown in Figure 2. It is currently accepted that atherosclerotic disease is an inflammatory process and not only mere excesses of accumulation of lipids. There is also mounting evidence that the tissue destruction observed in periodontal disease is caused primarily by a host inflammatory response, as opposed to the consequence of the destructive effect of the pathogens. Chronic infection may initiate atherosclerosis or interact with other risk factors to amplify the vessel inflammatory response.39 This response may be manifested by alteration of endothelial function or acceleration of atherosclerotic lesions. Acute infection may destabilize plaques or exert inflammatory and thrombotic effects on atherosclerotic plaques.39 Infection also may contribute to elevation of acute phase proteins, which, in turn, may modulate atherogenesis.38 Many studies have demonstrated an association between periodontal disease and acute-phase proteins, such as C-reactive protein and fibrinogen.38,40 Studies also have shown the presence of oral pathogens in arterial plaque. In the Haraszthy et al study41 of surgical specimens obtained during carotid endarterectomy, 44% of the 50 atheromas were positive for at least one of the target periodontal pathogens. In Beck et al’s study42 of carotid intima-medial thickness (IMT), participants with antibodies to specific periodontal pathogens had a great likelihood of having increased IMT. Of particular relevance to the theory that periodontal disease-induced inflammation could alter endothelial function is the recent report demonstrating improvement in endothelial function in patients after treatment of periodontal disease.43
Overall Strength of Evidence
Table 4 summarizes the overall strength of evidence according to the causal criteria for atherosclerotic disease. In summary, the overall strength of evidence for causal criteria for the relation between periodontal disease and atherosclerotic
disease is as follows: specificity is not important and is not established; the magnitude and consistency of the association is stronger for stroke; there is some initial evidence for dose response; consistency is low for IHD; time sequence has been established also with more evidence for stroke; and there is definitely biologic plausibility. Independence from confounding is also stronger for ischemic stroke and PAD.
Future Directions
Additional well-conducted prospective studies are needed to assess these associations in additional populations including developing countries, to evaluate mediators and to evaluate the role of genetic factors. The role of various inflammatory mediators (such as free radicals, cytokines, and prostaglandins) in both the modulation and interaction of periodontal disease with other disease states is ripe for further investigation. The evidence linking periodontal disease and IHD, especially for the independence from confounding criterion, would be enhanced greatly with randomized controlled trials. However, it is important to note that clinical trials would not be able to answer all the questions. Practical considerations, such as how many periodontal treatments to allocate to make the two groups sufficiently different and how long a follow-up period is feasible to enable accumulation of sufficient number of cardiovascular cases while limiting attrition, make this pursuit difficult. Clinical trials also would not be able to provide direct information on pathophysiology relating the systemic impact of periodontal disease (not treatment). More importantly, trials can compare only people with and without periodontal treatment, whereas observational studies can suggest means for primary prevention by comparing CVD risk between people with and without periodontal disease. Hence, a combination of observational and interventional studies are needed.
Conclusions
At the present time, there is insufficient but suggestive evidence for a possible causal relation between periodontal disease and atherosclerotic disease, with a little stronger evidence for stroke. If future studies show consistent associations, periodontal disease may be elucidated as an independent and potentially modifiable causal risk factor for atherosclerotic disease.
Acknowledgment
The authors would like to thank Dr. Oelisoa Mireille Andriankaja for helping fact-check the tables.
References
1. Fuster V, Moreno PR, Fayad ZA, et al. Atherothrombosis and high-risk plaque: part 1: evolving concepts. J Am Coll Cardiol. 2005;46(6): 937-954.
2. Tiong AY, Brieger D. Inflammation and coronary artery disease. Am Heart J. 2005;150(11): 11-18.
3. Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15(6): 403-413.
4. Chun YH, Chun KR, Olguin D, et al. Biological foundation for periodontitis as a potential risk factor for atherosclerosis. J Periodontal Res. 2005;40(1): 87-95.
5. Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66(11): 5337-5343.
6. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295-300.
7. Höfler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol. 2005;2:11.
8. Joshipura K, Ritchie C, Douglass C. Strength of evidence linking oral conditions and systemic disease. Compend Contin Educ Dent. 2000;21(suppl 30):12-23.
9. Beck JD, Couper DJ, Falkner KL, et al. The Periodontitis and Vascular Events (PAVE) pilot study: adverse events. J Periodontol. 2008;79(1):90-96.
10. Rothman KJ, Greenland S. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998.
11. Rothman KJ. Causes. Am J Epidemiol. 1976;104(6): 587-592.
12. Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction [comment in: BMJ. 1989;298(6687):1579-1580]. BMJ. 1989;298:779-781.
13. DeStefano F, Anda RF, Kahn HS, et al. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306(6879):688-691.
14. Hujoel PP, Drangsholt M, Spiekerman C, et al. Periodontal disease and coronary heart disease risk. JAMA. 2000;284(9): 1406-1410.
15. Joshipura KJ, Rimm EB, Douglass CW, et al. Poor oral health and coronary heart disease. J Dent Res. 1996;75(9): 1631-1636.
16. Beck J, Garcia R, Heiss G, et al. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(10 suppl):1123-1137.
17. Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6(1): 7-11.
18. Tuominen R, Reunanen A, Paunio M, et al. Oral health indicators poorly predict coronary heart disease deaths. J Dent Res. 2003;82(9):713-718.
19. Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1): 27-32.
20. Dietrich T, Jimenez M, Krall Kaye EA, et al. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. 2008;117(13):1668-1674.
21. Mattila KJ, Valtonen VV, Nieminen M, et al. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis. 1995;20(3):588-592.
22. Hujoel PP, Drangsholt M, Spiekerman C, et al. Pre-existing cardiovascular disease and periodontitis: a follow-up study. J Dent Res. 2002;81(3): 186-191.
23. Mendez MV, Scott T, LaMorte W, et al. An association between periodontal disease and peripheral vascular disease. Am J Surg. 1998;176(2):153-157.
24. Hung HC, Willett WC, Merchant A, et al. Oral health and peripheral artery disease. Circulation. 2003;107(8):1152-1157.
25. Howell TH, Ridker PM, Ajani UA, et al. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J Am Coll Cardiol. 2001;37(2):445-450.
26. Hung H, Joshipura K, Colditz G, et al. The association between tooth loss and coronary heart disease in men and women. J Public Health Dent. 2004;64(4):209-215.
27. Joshipura KJ, Hung HC, Rimm EB, et al. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34(1):47-52.
28. Geerts SO, Legrand V, Charpentier J, et al. Further evidence of the association between periodontal conditions and coronary artery disease. J Periodontol. 2004;75(9):1274-1280.
29. Kleinbaum DG, Sullivan KM, Barker ND. Activepi Companion Textbook. New York, NY: Springer; 2003.
30. Ajwani S, Mattila KJ, Narhi TO, et al. Oral health status, c-reactive protein and mortality—a 10 year follow-up study. Gerodontology. 2003;20(1):32-40.
31. Wu T, Trevisan M, Genco RJ, et al. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160(18):2749-2755.
32. Newell DJ. Errors in the interpretation of errors in epidemiology. Am J Public Health Nations Health. 1962;52:1925-1928.
33. Copeland KT, Checkoway H, McMichael AJ, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488-495.
34. Joshipura KJ, Douglass CW, Garcia RI, Valachovic R, Willett WC. Validity of a self-reported periodontal disease measure. J Public Health Dent. 1996;56(4): 205-212.
35. Pitiphat W, Garcia RI, Douglass CW, et al. Validation of self-reported oral health measures. J Public Health Dent. 2002;62(2): 122-128.
36. Joshipura KJ, Douglass CW, Willett WC. Possible explanations for the tooth loss and cardiovascular disease relationship. Ann Periodontol. 1998;3(1): 175-183.
37. Hujoel PP. Does chronic periodontitis cause coronary heart disease? A review of the literature. J Am Dent Assoc. 2002;133(Suppl):31S-36S.
38. Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321(7255): 199-204.
39. Kiechl S, Egger G, Mayr M, et al. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103(8):1064-1070.
40. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685-1695.
41. Haraszthy VI, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71(10): 1554-1560.
42. Beck JD, Elter JR, Heiss G, et al. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21(11): 1816-1822.
43. Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911-920.
About the Authors
Kaumudi Joshipura, BDS, MS, ScD
Professor of Epidemiology
University of Puerto Rico, School of Dentistry
San Juan, Puerto Rico
Adjunct Faculty
Harvard School of Dental Medicine
Harvard School of Public Health
Boston, Massachusetts
Juan Carlos Zevallos, MD
Associate Professor of Medicine and Public Health and Director
Endowed Health Service Research Center
University of Puerto Rico
San Juan, Puerto Rico
Christine Seel Ritchie, MD, MSPH
Associate Professor of Medicine
Birmingham–Atlanta Veterans Administration Geriatric Research, Education and Clinical Center (GRECC)
Birmingham, Alabama
University of Alabama at Birmingham
Department of Medicine
Birmingham, Alabama
Adjunct Faculty
University of Puerto Rico
School of Oral Health
San Juan, Puerto Rico