You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
During and subsequent to exodontia, periodontal, or implant surgery, hemorrhaging can occur from soft tissue and/or alveolar bone. The term hemorrhage denotes that blood has escaped from damaged blood vessels. Throughout a procedure (eg, tooth extraction) or after flap closure, digital pressure with gauze usually results in hemostasis. However, during surgery or postoperatively, bleeding may occur that requires measures beyond gauze tamponade and suturing. This article addresses management of hemorrhaging associated with exodontia, periodontal, and implant surgeries among patients without bleeding disorders (eg, hemophilia). Background information is given to enhance understanding of physiological events and anatomical structures associated with hemorrhage during frequently executed dental surgical procedures.
Types of Hemorrhage
Three types of bleeding can occur during surgery, and their occurrence depends on the kinds of blood vessels that are damaged: arterial, venous, and capillary.1 Arterial bleeding results from damage to an artery, and hemorrhage is pulsatile, brisk, and bright red in color. Venous bleeding has a darker hue and blood flows in a smooth stream. Capillary hemorrhage is characterized by oozing from the area and no bleeding point can be discerned.
Bleeding can be described as primary, reactionary, or secondary.1,2 Primary hemorrhage happens at the time of injury. Reactionary hemorrhage is that which occurs immediately after surgery (<24 hours) (Figure 1). Bleeding that is delayed (>24 hours) is called secondary hemorrhage and may be caused by clot dislodgement, trauma, or infections.1
Patient Assessment Before Surgery
A physical examination before oral surgery should be conducted. The neck, skin, and mucosal surfaces should be inspected for abnormal findings associated with hepatomegaly, splenomegaly, etc.1 Hepatic insufficiency can manifest signs of jaundice or telangiectasias. Individuals with splenomegaly can have yellowing of the skin and whites of the eyes. It is prudent to take a patient history specifically related to bleeding issues. Most abnormal conditions of vessels and platelets are acquired (eg, thrombocytopenia), while serious coagulation disorders are hereditary (Table 1).3 A history of surgery that did not cause abnormal bleeding is a reasonable sign that there are no hereditary coagulation disorders.
Based on a patient's medical history or physical appearance, if there are concerns regarding the possibility that the patient may have a bleeding disorder, a variety of laboratory tests can be ordered, including bleeding time, platelet function assay, platelet count, prothrombin time, and partial thromboplastin time.4 Individuals with specific issues (eg, hemophilia) may need to be administered clotting factors before surgery and may need to be treated in a hospital environment.1
Blood pressure and pulse rate should be recorded prior to surgical procedures. Elevated blood pressure can lead to excessive bleeding during surgical procedures, and it is recommended that elective dental procedures be avoided in patients whose blood pressure is above 180/110 mm Hg.5
Suspending Medications If Necessary
Prior to surgery it may be necessary for the patient to stop taking certain drugs to preclude their effects on bleeding. The following guidelines for common medications are suggested:
Aspirin irreversibly affects platelets, but usually it is unnecessary to avoid taking aspirin before routine dental surgery.6,7 For a procedure such as a sinus lift, however, the authors suggest having the patient stop aspirin ingestion 5 to 7 days before surgery, because the application of compression may not always be effective within the sinus due to its limited access.
Clopidogrel (Plavix®) irrevocably alters platelets but typically does not need to be suspended 5 to 7 days before routine dental surgery.6,7The same precaution noted above for aspirin with respect to sinus lifts, however, applies to Plavix ingestion as well as other antiplatelet aggregation drugs, including ticlopidine (Ticlid®), prasugrel (Effient®), and ticagrelor (Brilinta®).
Warfarin (Coumadin®) may or may be not be stopped or decreased, with a physician's guidance, 3 days prior to surgery.6,7 A systematic review concluded that the optimal international normalized ratio (INR) value for dental surgical procedures is 2.5, because this reduces the risk of bleeding or thromboembolism. However, the review stated that minor procedures, such as biopsies, non-complicated tooth extraction, or periodontal surgery, can be done safely with an INR lower than 4.8 This concurs with the American Dental Association's (ADA) assessment that there is strong evidence that warfarin does not need to be discontinued prior to routine surgical procedures (Table 2).9
Dabigatran (Pradaxa®), rivaroxaban (Xarelto®), and apixaban (Eliquis®): Abstaining from these anticoagulant drugs for 2 days is required for them to have no effect. Sometimes these medications may be stopped for 1 day prior to surgery or not stopped, depending on the physician's assessment of risk that drug suspension might pose for the patient. Half-life of these drugs is 12 to 17 hours for Pradaxa, 7 to 13 hours for Xarelto, and 8 to 13 hours for Eliquis.10
According to the ADA, anticoagulation or antiplatelet therapy does not need to be altered prior to dental surgical intervention for most patients (Table 2).9 Strong evidence exists for not stopping medications such as antiplatelet agents, but there is only limited evidence for this recommendation with respect to newer direct-acting oral anticoagulants (DOACs) (Table 2).9 In this regard, a recent review by Fortier et al concluded there is insufficient data to establish an evidence-based treatment protocol for dental surgical patients taking the newer anticoagulants.11 Therefore, treatment protocols for patients taking DOAC drugs need to be based on the patient's health status and type of procedure(s) to be performed. Bleeding risks associated with treatment of patients on anticoagulants have been discussed in various publications.7,12-15
Herbal drugs and vitamins that may increase bleeding time include garlic, ginseng, gingko biloba, ginger, and vitamin E. Stopping these agents a week before surgical procedures may be beneficial.16
Vascularity in Different Regions of the Oral Cavity
Because major arteries and veins are located deep within the connective tissue of the oral cavity they typically are not damaged during routine dental surgical procedures.17,18 Nonetheless, to understand various bleeding scenarios that may be encountered when performing such surgical procedures, clinicians must be cognizant of the various blood vessels that potentially can be damaged (Figure 2).
Blood supply to the mandible: The external carotid artery gives off blood vessels in the neck, specifically the lingual and facial arteries. The lingual artery provides the sublingual artery that is found coronal to the mylohyoid muscle, and the facial artery supplies the submental artery, which is located inferior to the mylohyoid muscle. These vessels provide vascularity to the floor of the mouth and the tongue. The lingual gingiva derives blood supply from the sublingual artery, and the buccal gingiva gets its vascular supply from the buccal artery.
Vascularity in the maxilla: The external carotid artery gives rise to the maxillary artery, which provides a variety of branches important to the dental surgeon. In the palate, the greater palatine artery emerges from the greater palatine foramen and courses anteriorly in the groove located between the horizontal and vertical walls of the palatal vault, and the nasopalatine artery emerges from the nasopalatine foramen lingual to the maxillary incisors. The lingual gingival tissues receive vascularity from palatal vessels, and the buccal gingiva is supplied by the buccal artery and the posterior, middle, and anterior superior alveolar arteries.
Hemostasis and Management of Intraoral Bleeding
If blood vessel injury occurs, hemostasis needs to be achieved. Hemostasis comprises three main steps: vasoconstriction, platelet plug formation, and coagulation.19 Initially, the endothelium of damaged blood vessels releases vasoconstrictive endothelins (chemicals) to reduce blood flow. Then, there is mechanical blockage of the vessel defects by platelets that attach to the exposed collagen (platelet adhesion). Chemical mediators are released by the platelets, and platelet aggregation occurs forming a platelet plug. The clotting cascade resulting in coagulation consists of an extrinsic and intrinsic pathway. These pathways join in a common pathway where thrombin converts to fibrinogen, then to fibrin, which then creates the final clot (Figure 3).20
The potential for increased bleeding that may require the management of hemorrhaging can be associated with any surgical procedure. Various scenarios may necessitate bleeding control measures:
Tooth extraction: When a tooth is extracted and the apical blood vessels are large there may be an extreme hemorrhagic response. Suction should be applied so that the precise source of the bleeding can be determined. If possible, an anesthetic needle should be put into the apical foramen of the socket and local anesthetic with epinephrine should be injected. Alternatively, cautery could be applied if available. Minor cauterization of the bone does not impede healing. Another technique involves saturating a piece of gauze with local anesthetic/epinephrine, rolling it, and then pushing it into the socket and holding it in place with a periosteal elevator.2,21 The authors have found these three methods to be effective in controlling a hemorrhagic socket.
Two other bleeding control techniques can also be used: bone can be crushed with an instrument, or bone graft material can be inserted and forced into place occluding nutrient canals in the bone.2,21 Generally speaking, bleeding should be stopped before placement of the graft material.
An important factor in the cessation of bleeding is time (bleeding time is 1 to 9 minutes).22 Other commonly used antihemorrhagic agents can be applied to a bleeding site; these include but are not limited to Surgicel®, Gelfoam®, thrombin, and Avitene™ (Table 3).2,19,23
Periodontal surgery: Several hemorrhagic scenarios may occur during periodontal surgery. Bleeding from the bone can be managed as described above for extraction sockets. Bleeding from the soft tissue or a flap usually can be managed with digital pressure or local anesthetic with epinephrine injected into the tissue or a combination of both techniques. Cautery is also useful. If an arteriole is squirting, pressure should be applied and the flap may be clamped with a hemostat.
When harvesting a connective tissue graft from the palate, a branch of the greater palatine artery or the artery itself may become severed. Clinicians should note that the palatal vault is classified as shallow (7 mm height), medium (12 mm height), or tall (17 mm height).24 When harvesting a connective tissue graft, the clinician should get no closer than 2 mm from the greater palatine artery to avoid damaging the artery,24 which runs in a groove where the horizontal and vertical aspects of the palate merge.18 If this artery is damaged and hemorrhaging results, the following steps can be taken, as per the authors' experience: use suction, apply pressure, inject anesthetic with epinephrine, clamp the flap, cauterize, and if the blood vessel is visible, ligate it. If the blood vessel is not visible, perform suturing using a FS-1 needle, which is a large needle; start at the site of the wound and insert the needle to the bone, loop around the blood vessel, and tie a knot. This is continued every 3 mm, working toward the greater palatine foramen.
Implant Surgery: Potential Bleeding Issues
Nasopalatine canal: When the palatal tissue needs to be reflected over the nasopalatine canal to facilitate bone grafting in preparation for a dental implant, the contents of the canal, including the nasopalatine artery, should be enucleated (Figure 4).25,26 As the tissue is removed, there is often a hemorrhagic response. The procedure to control bleeding is similar to management of a hemorrhaging extraction socket.
Osteotomy: Flap refection may expose small blood vessels in the bone (nutrient canals), which can exude blood and vertically pump blood up several millimeters. This may be managed with digital pressure, injection of local anesthetic with epinephrine into the nutrient canal, or thermal cautery.When an osteotomy is drilled, a large nutrient canal may be encountered and pumping of blood (possibly several millimeters high) from the bone may occur. After the drill reaches its proper length, it is removed, and a guide pin should be inserted into the osteotomy to obtund bleeding. After use of each additional drill, the corresponding guide pin should be inserted to control bleeding until the procedure is finished.27 The implant will act as a "cork in the bottle" and obtund all bleeding (Figure 5). If a socket is still bleeding after an immediate implant placement, it may be handled as described earlier for tooth extraction socket management.
Sinus lift: Extreme bleeding may occur for various reasons when creating a lateral wall osteotomy. A blood vessel that traverses along the Schneiderian membrane can be damaged, or an intraosseous artery in the lateral wall of the sinus might be cut. Controlling hemorrhage from the artery on the membrane can be achieved by placing gauze saturated with anesthetic solution (1/50,000 epinephrine) onto the artery (Figure 6).27,28 Bleeding from the osseous wall will need either direct pressure with an instrument (eg, a hemostat), or it could be touched with a cautery unit. If the lateral window has been created, an alternate way to address an intraosseous arterial bleeder is to elevate the membrane and clamp the bone with a hemostat to crush the bone, thereby occluding the bleeding blood vessel (Figure 7), or cautery on the bleeding site can be used. It should be noted that in 20% of cases intraosseous arteries are less than 16 mm from the crest of the ridge and may present a complication during lateral window preparation.29
Inadvertent perforation of the lingual cortical plate: If bleeding is induced in the floor of the mouth due to a lingual plate perforation when creating an osteotomy, anesthetic solution with epinephrine can be used to cause vasoconstriction and firm pressure should be applied with gauze to the floor of the mouth.27 When performing gauze tamponade, one thumb is placed on the floor of the mouth and the index finger is placed outside the mouth, and prolonged pressure is applied. Ligation of the hemorrhaging blood vessel is the preferred therapy and provides the most reliable result, although this may be difficult to perform.27,30 After hemorrhaging is controlled, patients should be carefully monitored to determine if they should be relocated to a hospital setting for monitoring and possible airway management. If bleeding persists, the clinician should call for help (a medical center), because direct ligation of the blood vessel may be required. If hemorrhaging continues and a sublingual hematoma develops, an airway crisis can develop and the patient might require intubation.27
Blood Loss During Dental Surgery
An average person has around 5000 ml of blood (five liters); one pint contains 473 ml.31 The authors know of no studies that have addressed the amount of hemorrhage that occurs during implant procedures or alveolar ridge or sinus augmentations. After periodontal surgery, which typically lasts less than 2 hours, blood loss is usually <125 ml (maxilla 110 ml, mandible 151 ml).32 It would be expected that less blood would be lost during a simple implant procedure, but this can vary depending on numerous factors (eg, extent of surgery, health of tissues, etc). If a patient's blood pressure drops 20 mg, or blood loss is >500 ml, or the patient's heart rate increases by 20%, intravenous solutions may be needed to increase blood pressure.33
The amount of blood hemorrhage can be affected by the concentration of epinephrine in an anesthetic, causing vasoconstriction. Blood loss after a sextant of periodontal surgery using 1/50,000 versus 1/100,000 concentration of epinephrine with lidocaine was reported to be <120 ml versus <246 ml, respectively.34
If a blood vessel in the floor of the mouth (eg, sublingual artery) is severed, hemorrhage can result in up to 420 ml loss of blood in 30 minutes.30 This was calculated for an artery 2 mm in diameter with an estimated blood flow of 0.2 ml per beat (70 beats per minute x 0.2 ml = 14 ml/minute, 14 ml/minute x 30 minutes = 420 ml of blood loss).30
Postoperative Complications Related to Bleeding
Hemorrhaging after suturing: Although an infrequent occurrence, bleeding that had been under control may begin to appear through the incision line after a flap is sutured. This may be due to a rebound effect occurring, whereby the vasoconstriction provided by the epinephrine is wearing off and there is vasodilation of blood vessels. This event usually can be corrected with the application of pressure for 10 minutes and injection of additional local anesthetic with epinephrine. Importantly, the patient should not be dismissed until all hemorrhaging has stopped. If hemorrhaging persists, the flap should be reopened and the bleeding site detected. Thermal cautery can be used or hemostatic agents (eg, Surgicel, Gelfoam, Avitene) applied, and the site should be resutured and monitored to ensure all bleeding has stopped before the patient is dismissed.
Postoperative ooze (reactionary hemorrhage): If after leaving the office patients experience postoperative bleeding at home, they should be instructed to apply moistened gauze under digital pressure over the surgical site for 10 minutes. If that does not control the hemorrhage, they should do it again for another 10 minutes. As a last resort, a moistened green or black tea bag wrapped in gauze could be applied under digital pressure on the bleeding site. These types of tea bags contain tannic acid, which enhances the clotting mechanism.35 If this fails to halt the hemorrhage the patient should be seen to correct the situation. The patient should not be left in this condition during the night, because during sleep if the ooze continues the patient may swallow an excessive amount of blood, which can result in vomiting.
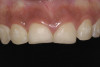
Liver clot: Rarely, post-extraction liver clots form (Figure 1). However, when they do occur, it is due to incomplete fibrin clotting, and they manifest as a slowly developing, red-brown mass. If a patient develops a liver clot and is not in the office, he or she may have difficulty controlling the bleeding. The patient should be instructed to wipe away the clot with a piece of gauze and apply pressure for 10 minutes. If this does not control the bleeding, the patient should return to the office and the clinician should wipe the clot away, inject the bleeding sites with anesthetic containing 1/50,000 epinephrine, curette out the oozing fibrin clot, and resuture the area.36 Occasionally, cautery may be required to control bleeding in this type of situation. The patient should bite on gauze for 10 minutes to ensure an adequate fibrin clot has formed.
Ecchymosis and color changes of tissues: As the effect of epinephrine wears off after dental surgery, vasodilation of blood vessels ensues. If postoperative bleeding occurs, blood can advance along fascial planes and cause an ecchymosis whose pattern can vary. Tissue discoloration may appear adjacent to a surgical site or, due to gravity, extend down as far as the pectoral muscles (Figure 8). The chronology of color change related to hemoglobin breakdown is predictable: initially the ecchymosis appears reddish, in several hours it can become black and blue, by day 6 its color changes to green (hemoglobin converted to biliverdin), and by day 8 to 9 it may be yellowish-brown (bilirubin present). In general, the body resorbs hemoglobin and hemoglobin by-products in 2 to 3 weeks.37 The finding of an ecchymosis does not typically require therapy, and verbal and written postoperative instruction should inform the patient that these sequelae are not necessarily a problem.
Conclusion
This article discussed clinical techniques that dentists can use to manage bleeding when performing common surgical procedures. Successfully executed surgery is based on careful treatment planning and attention to detail.
About the Authors
Gary Greenstein, DDS, MS
Clinical Professor, Department of Periodontics, College of Dental Medicine, Columbia University; Private Practice, Surgical Implantology and Periodontics, Freehold, New Jersey
John Cavallaro, DDS
Former Clinical Professor, Department of Prosthodontics, College of Dental Medicine, Columbia University; Private Practice, Surgical Implantology and Prosthodontics, Brooklyn, New York
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Singh J, Santosh Reddy G, Allwyn Meshack R, et al. An overview in management of intraoral bleeding. Int J Clin Dent Sci. 2012;3(2):39-43.
2. McCormick NJ, Moore UJ, Meechan JG. Haemostasis. Part 1: The management of post-extraction haemorrhage. Dent Update. 2014;41(4):290-296.
3. Moake JL. Overview of Coagulation Disorders. MSD Manual website. Updated January 2020. https://www.msdmanuals.com/professional/hematology-and-oncology/coagulation-disorders/overview-of-coagulation-disorders. Accessed November 2, 2020.
4. Hayward CP, Moffat KA. Laboratory testing for bleeding disorders: strategic uses of high and low-yield tests. Int J Lab Hematol. 2013;35(3):322-333.
5. Southerland JH, Gill DG, Gangula PR, et al. Dental management in patients with hypertension: challenges and solutions. Clin Cosmet Investig Dent. 2016;8:111-120.
6. McBeth PB, Weinberg JA, Sarani B, et al. A surgeon's guide to anticoagulant and antiplatelet medications part one: warfarin and new direct oral anticoagulant medications. Trauma Surg Acute Care Open. 2016;1(1):e000020.
7. Yeung LYY, Sarani B, Weinberg JA, et al. Surgeon's guide to anticoagulant and antiplatelet medications part two: antiplatelet agents and perioperative management of long-term anticoagulation. Trauma Surg Acute Care Open. 2016;1(1):e000022.
8. Pototski M, Amenábar JM. Dental management of patients receiving anticoagulation or antiplatelet treatment. J Oral Sci. 2007;49(4):253-258.
9. American Dental Association. Oral Health Topics. Oral Anticoagulant and Antiplatelet Medications and Dental Procedures. ADA website. Updated September 14, 2020. https://www.ada.org/en/member-center/oral-health-topics/oral-anticoagulant-and-antiplatelet-medications-and-dental-procedures. Accessed November 2, 2020.
10. Wynn RL. The New Anticoagulants: Bleeding Risks in Dental Management. Wolters Kluwer website. https://www.wolterskluwercdi.com/blog/new-anticoagulants-bleeding-risks-dental-management/. Accessed January 20, 2020.
11. Fortier K, Shroff D, Reebye UN. Review: an overview and analysis of novel oral anticoagulants and their dental implications. Gerodontology. 2018;35(2):78-86.
12. Wahl MJ. The mythology of anticoagulation therapy interruption for dental surgery. J Am Dent Assoc. 2018;149(1):e1-e10.
13. Miller CS. A perspective on "The mythology of anticoagulation interruption for dental surgery." J Am Dent Assoc. 2018;149(1):3-6.
14. Daly C. Treating patients on new anticoagulant drugs. Aust Prescr. 2016;39(6):205-207.
15. Lusk KA, Snoga JL, Benitez RM, Sarbacker GB. Management of direct-acting oral anticoagulants surrounding dental procedures with low-to-moderate risk of bleeding. J Pharm Pract. 2018;31(2):202-207.
16. Abebe W. Review of herbal medications with the potential to cause bleeding: dental implications, and risk prediction and prevention avenues. EPMA J. 2019;10(1):51-64.
17. Kamrani P, Sadiq NM. Anatomy, head and neck, oral cavity (mouth). In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020.
18. Vascular supply to the oral cavity. In: Norton NS, ed. Netter's Head and Neck Anatomy for Dentistry. Philadelphia, PA: Saunders; 2007:372-378.
19. Kumar S. Local hemostatic agents in the management of bleeding in oral surgery. Asian J Pharm and Clin Res. 2016;9(3):35-41.
20. Chaudhry R, Usama SM, Babiker HM. Physiology, coagulation path-ways. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020.
21. Kumbargere Nagraj S, Prashanti E, Aggarwal H, et al. Interventions for treating post-extraction bleeding. Cochrane Database Syst Rev. 2018;3(3):CD011930.
22. Mielke CH. Measurement of the bleeding time. Thromb Haemost. 1984;52(2):210-211.
23. Mani A, Anarthe R, Kale P, et al. Hemostatic agents in dentistry. Galore International Journal of Health Sciences and Research. 2018;3(4):40-46.
24. Reiser GM, Bruno JF, Mahan PE, Larkin LH. The subepithelial connective tissue graft palatal donor site: anatomic considerations for surgeons. Int J Periodontics Restorative Dent. 1996;16(2):130-137.
25. Singhal MK, Dandriyal R, Aggarwal A, et al. Implant placement into the nasopalatine foramen: considerations from anatomical and surgical point of view. Ann Maxillofac Surg. 2018;8(2):347-351.
26. Cavallaro J, Tsuji S, Chiu TS, Greenstein G. Management of the nasopalatine canal and foramen associated with dental implant therapy. Compend Contin Educ Dent. 2016;38(6):367-372.
27. Greenstein G, Cavallaro J, Romanos G, Tarnow D. Clinical recommendations for avoiding and managing surgical complications associated with implant dentistry: a review. J Periodontol. 2008;79(8):1317-1329.
28. Greenstein G, Cavallaro J. Mapping the maxillary sinus. Decisions in Dentistry. 2016;2(12):12-17.
29. Elian N, Wallace S, Cho SC, et al. Distribution of the maxillary artery as it relates to sinus floor augmentation. Int J Oral Maxillofac Implants. 2005;20(5):784-787.
30. Flanagan D. Important arterial supply of the mandible, control of an arterial hemorrhage, and report of a hemorrhagic incident. J Oral Implantol. 2003;29(4):165-173.
31. Lundsgaard-Hansen P. Treatment of acute blood loss. Vox Sang. 1992;63(4):241-246.
32. Baab DA, Ammons WF Jr, Belinsky H. Blood loss during periodontal flap surgery. J Periodontol. 1977;48(11):693-698.
33. Gladfelter IA Jr. A review of blood transfusion. Gen Dent. 1988;36
(1):37-39.
34. Buckley JA, Ciancio SG, McMullen JA. Efficacy of epinephrine concentration in local anesthesia during periodontal surgery. J Periodontol. 1984;55(11):653-657.
35. Stal L. Understanding how a tea bag can assist with bleeding control after a dental extraction. Lugansk Stal website. March 2, 2017. http://luganskstal.com/2017/03/02/understanding-how-a-tea-bag-can-assist-with-bleeding-control-after-a-dental-extraction/. Accessed November 2, 2020.
36. Cavallaro J, Greenstein B, Greenstein G. Extracting teeth in preparation for dental implants. Dentistry Today. 2014;33(10):92-100.
37. Timmons J. The Colorful Stages of Bruises: What's Going on in There? Healthline website. Updated March 8, 2019. https://www.healthline.com/health/bruise-colors. Accessed November 2, 2020.