Henry Schein, Inc. (Nasdaq: HSIC) announced today its latest virtual resource to help dental and medical practice owners navigate the COVID-19 pandemic. “Best Practices for Better Practices: A Henry Schein Wellness Symposium,” will launch on Thursday, April 9 at 10:00 a.m. EST. To register, click here.
As part of the Company’s continued offering of educational resources customers can rely on amid the outbreak, Henry Schein’s virtual symposium will present best practices on a broad range of topics from experts across a spectrum of specialties and backgrounds, including:
What’s Next With the Outbreak? – Bruce Lieberthal, Chief Innovation Officer, Henry Schein, with Dr. Amesh Adalja, Senior Scholar, Johns Hopkins University Center for Health Security;
Enhancing Team Morale and Maintaining Emotional Stability While Working Remotely – Barbara Fisenne, Vice President, Global Organizational Development, Henry Schein, with Dr. Kerry Sulkowicz, Managing Principal, Boswell Group LLC;
Maintaining Practice Financial Health – Natalie Westfall, Executive Director, Henry Schein Financial Services, with Mark Rosen, Partner, Rosen & Associates, LLP;
Patient Communications During and After COVID-19 – Andrea Gallimore, Product Marketing Manager, Henry Schein One, with Lois Banta, CEO, Banta Consulting;
Improving Patient Care with Telehealth Using Clinical Decision Support – Michael Casamassa, Vice President of Solutions and Planning, Henry Schein Medical, with Dr. Art Papier, CEO, VisualDx;
Maximizing Time During Office Closings – Scott Jackson, Senior Director, Ambulatory Surgery Center Segment, Henry Schein Medical, with Gayle Evans, President, Continuum Healthcare Consultants, and Sandra Sommerman, Senior Project Manager, Continuum Healthcare Consultants.
“Henry Schein has a long record of assisting our customers during difficult times,” said Stanley M. Bergman, Chairman of the Board and Chief Executive Officer of Henry Schein. “This virtual symposium is another example of how our customers can rely on us to help health care practices manage through a challenging moment. We are committed to doing all we can to ensure that our customers emerge strong from the pandemic, and the best practices detailed in this symposium is a part of that effort.”
In addition to the symposium, Henry Schein provides a rich selection of practical information to help customers with the wide range of issues presented by the pandemic, which can be found on the Coronavirus (COVID-19) Resource Center. Customers can also find webinars on timely topics, and other helpful resources from Henry Schein and its partners on the Coronavirus (COVID-19) Education Center.
To learn more about what Henry Schein is doing to address this unprecedented situation and the actions the Company is taking to get more product into the hands of those who need it most – health care workers – please visit www.henryschein.com/COVID19update.
To register for the virtual symposium, visit https://henryscheindigital.com/covid-19-wellness-symposium.
About Henry Schein, Inc.
Henry Schein, Inc. (Nasdaq: HSIC) is a solutions company for health care professionals powered by a network of people and technology. With more than 19,000 Team Schein Members worldwide, the Company's network of trusted advisors provides more than 1 million customers globally with more than 300 valued solutions that improve operational success and clinical outcomes. Our Business, Clinical, Technology, and Supply Chain solutions help office-based dental and medical practitioners work more efficiently so they can provide quality care more effectively. These solutions also support dental laboratories, government and institutional healthcare clinics, as well as other alternate care sites.
Henry Schein operates through a centralized and automated distribution network, with a selection of more than 120,000 branded products and Henry Schein private-brand products in stock, as well as more than 180,000 additional products available as special-order items.
A FORTUNE 500 Company and a member of the S&P 500® index, Henry Schein is headquartered in Melville, N.Y., and has operations or affiliates in 31 countries. The Company's sales from continuing operations reached $10.0 billion in 2019, and have grown at a compound annual rate of approximately 13 percent since Henry Schein became a public company in 1995.
For more information, visit Henry Schein at www.henryschein.com, Facebook.com/HenrySchein, and @HenrySchein on Twitter.




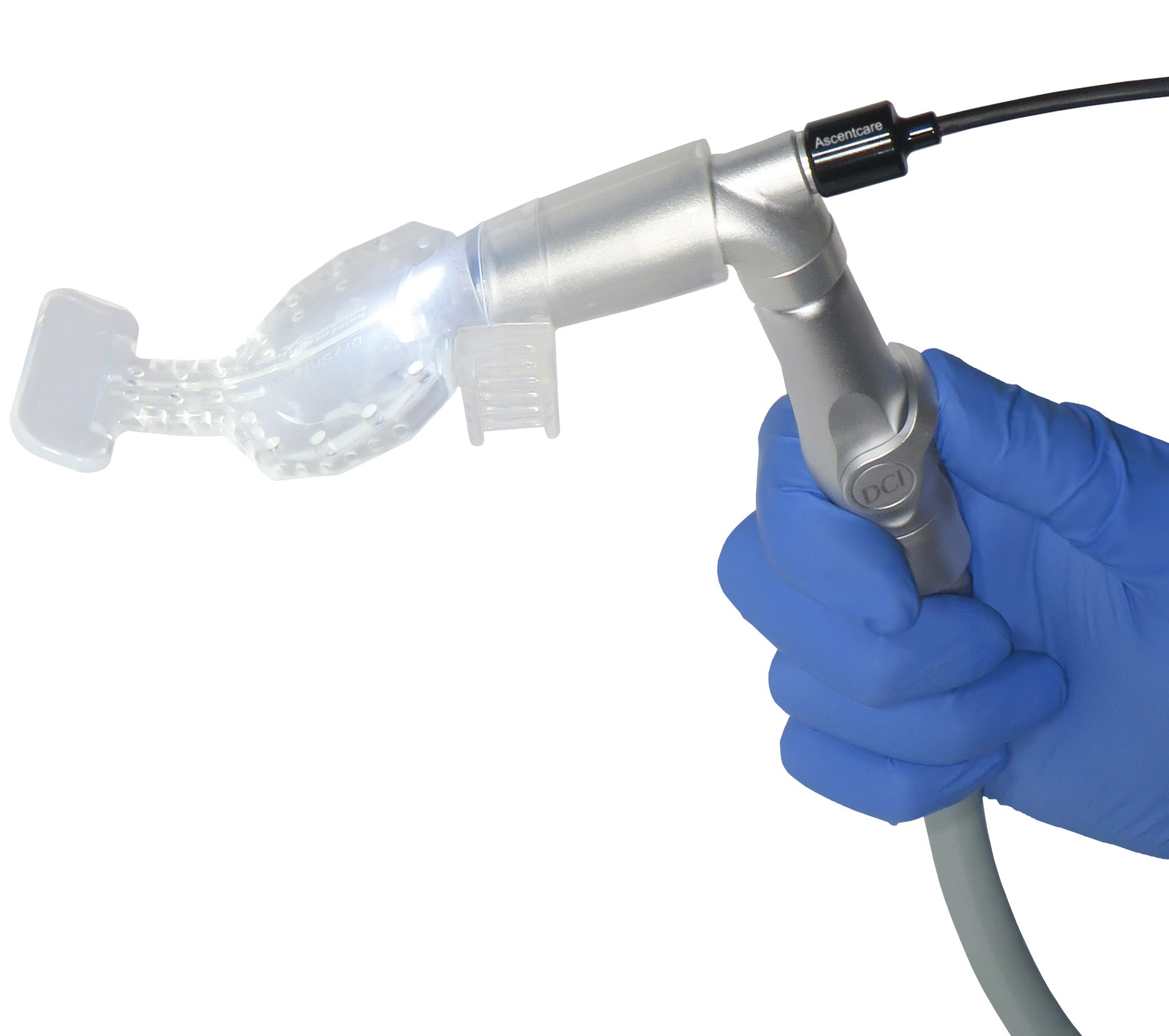 Ascentcare Dental Products, a dental products company focused on the creation of innovative dental technologies, has recently announced the release of their newest product, an illuminated HVE adapter for isolation mouthpieces that helps to remove 90% of infectious aerosols.
Ascentcare Dental Products, a dental products company focused on the creation of innovative dental technologies, has recently announced the release of their newest product, an illuminated HVE adapter for isolation mouthpieces that helps to remove 90% of infectious aerosols.
 National Dentex Labs (NDX) announced today that it is pleased to welcome Mr. David Waltzer as its new Chief Financial Officer. Mr. Waltzer will be responsible for all financial functions including accounting, audit, treasury, and corporate financial planning as well as acquisition integration activities.
National Dentex Labs (NDX) announced today that it is pleased to welcome Mr. David Waltzer as its new Chief Financial Officer. Mr. Waltzer will be responsible for all financial functions including accounting, audit, treasury, and corporate financial planning as well as acquisition integration activities.