You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Although dentistry has long been digitalized on the laboratory side, the clinical practice side has not kept pace and is still permeated by analog treatment planning and procedures. Today, conventional imaging techniques, such as panoramic and periapical radiographs, are decreasingly used as the sole tools for planning implant surgery because they do not provide adequate 3-dimensional anatomical information or enable 3D planning. The American Academy of Oral and Maxillofacial Radiology and other organizations have acknowledged the benefits of cross-sectional imaging for implant patients; however, a recent statement from the American Academy of Periodontology suggests that dental healthcare professionals should only consider using cone-beam computed tomography (CBCT) when they expect that the diagnostic information yielded will lead to better patient care, enhance patient safety, and ultimately facilitate a more predictable, optimal treatment outcome.1 This statement does not explicitly support the use of CBCT imaging as a standard for care for patients requiring dental implants. When an appropriate field of view is selected, along with an appropriate scan or exposure time, voxel size, and voltage, CBCT scanners offer lower radiation exposure.2 Surgically focused clinicians recognize the planning benefits achieved through the visualization of important anatomic structures and ridge architecture. CBCT imaging is the primary diagnostic tool used in virtual planning for computer-guided (ie, static) and computer-navigated (ie, dynamic) surgery.
Intraoral Scanning with CBCT
Based on reports of high precision and accuracy, digital impression taking, or intraoral optical scanning, has recently increased in popularity.3,4 Furthermore, the fusion of an intraoral scan's digital data with CBCT scan data has resulted in important advances for implant surgery, including enhanced diagnosis and treatment planning and improved communication between clinical team members, laboratory technicians, and patients. Preoperative visualization of the bone architecture can allow for more precise implant placement and more appropriate implant size selection for the available volume of bone, which may reduce the augmentation requirements and result in less complex surgery. When a flapless approach is possible, the surgery can become less invasive, reducing morbidity.
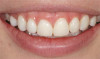
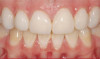
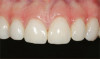
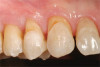
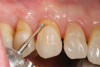
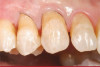
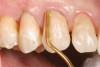
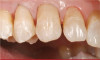
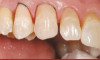
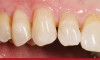
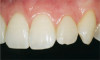
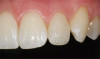
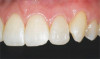
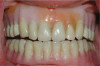
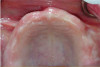
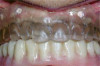
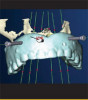
The fusion of intraoral scan data and CBCT data also permits prefabricated provisional prostheses to be planned where appropriate or desired. These can include prostheses used for tissue guidance, such as customized healing abutments coupled with fixed or removable restorations, or those used to adjust for minor deviations between planned and executed implant positions, such as fixed restorations with indexing methods (Figure 1 through Figure 8).
When compared with traditional impression-taking methods, intraoral scanning offers easier repeatability for the localized capture of relevant areas.- During surgery, this means that the introduction of impression materials into an open surgical site can be avoided, and when intraoral scanning is used post-integration, multiple impression retakes can be avoided by selectively scanning deficient regions.
Another factor is patient preference. Al--though both digital and analog methods of impressioning present their own unique challenges, patients often comment that the digital impression procedure is more appealing. A recent randomized controlled trial involving 1-year follow-up of restored single implants concluded that there were no significant differences in clinical or radio-graphic outcomes between the two impression methods; however, the digital workflow was preferred by patients and associated with a reduction in active treatment time and cost.5
In a prospective clinical crossover trial comparing time in digital and conventional workflows for the generation of implant crowns, Joda and Bragger concluded that the digital workflow was more time-efficient in terms of both clinical chairtime and laboratory manufacturing steps when intraoral scanning was combined with CAD/CAM technology.6 In a subsequent study by Joda and colleagues, 76% of dental students and 52% of dentists indicated a preference for intraoral scans over conventional impressions.7
The ability to perform virtual monitoring is another important benefit. Sequential CBCT scans are used in research to monitor various aspects of bone changes relating to implant treatment. In the same vein, longi--tudinal soft-tissue and dental structure monitoring via sequential intraoral scans may become a new standard in case documentation for clinical trials. Intraoral scanning has the potential to not only impact research, but also shape clinical practice and emerge as a more accurate method of monitoring changes in the volume and level of soft-tissue around teeth and implants as well as changes to teeth and restorations resulting from parafunction or disease states (Figure 9 through Figure 15). The value of a digital workflow that incorporates intraoral scanning and CBCT scanning has been documented in many publications.8-12 The surgical benefits include enhanced diagnosis and planning, improved communication, the possibility for simpler and less invasive surgery, and the ability to use prefabricated restorations for immediate tissue guidance. The restorative benefits include creating archivable data for repairs and remakes should transitional restoration components fail during the integration period, eliminating the disinfection steps required in procedures associated with impressions, reducing the need for cement-retained restorations by enabling screw-retained restorations through idealized implant positioning, providing virtual shade measurements for both pro-visional and definitive restorations, and im-proving prosthetic outcomes by facilitating virtual restoration before surgical treatment is carried out.
When analog impressions are made, labora-tories generally scan them (or the casts obtained from them) to convert their information into a digital format. The use of optical scanners to capture soft tissue and other dental structures has many applications in implant, surgical, and restorative procedures. Although photographs and videos are invaluable for treatment planning and case documentation, the emergence of 3D planning programs will likely overshadow the role of videography.
Planning and Design
The digital treatment planning process is carried out by merging the 3D radiographic image data with the optical scan data and then using planning software to preview the desired implant positions relative to the prosthetic plan.
Two-dimensional (Figure 16 through Fig-ure 18) and now, 3D (Figure 19 through Figure 22) smile designs drive virtual implant and restoration planning from both esthetic and functional perspectives. Face scanning links the intraoral condition to the face, which enhances esthetic and functional planning. When merged with intraoral scan data and CBCT scan data, a face scan can improve implant planning and esthetics as well as help coordinate orthodontic or orthognathic treatment decisions as they relate to esthetics and function.13,14 The result is improved communication between everyone involved-clinicians, technicians, and the patient.
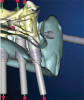
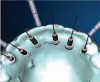
Conventional surgical templates (Figure 23 and Figure 24), which reproduce the desired dental reconstruction with rudimentary drilling entry points, do not control an osteo-tomy preparation in angle and in depth as accurately as stereolithographic surgical guides (Figure 25 through Figure 28).15,16 The use of 3D-printed surgical guides and provisional restorations, followed by the milling of definitive restorations, results in increased accuracy in the angle and depth of implants, faster laboratory production, and a reduction in material failures associated with analog processes.17,18 Innovators and early adopters have recognized the practical benefits of incorporating technologies that minimize analog planning and treatment protocols, whether they are carried out on-site, provided by mobile services, or outsourced to planning centers. Virtual planning of the restoration drives implant positioning and defines the required hard- and soft-tissue modifications (Figure 29 through Figure 35).
Digital Implant Surgical Workflows
Practice economics and hardware and software costs may stop some clinicians from venturing into the digital workflow. Clinicians who are against the use of guided planning and surgery may claim that "guides cost too much to use them," or "to remain competitive today, costs have to be reduced, so guided surgery does not work for my patient population." CBCT combined with intraoral scanning is key to integrating a successful digital workflow, reducing clinician stress, and improving practice profitability by minimizing the actual patient chairtime, which allows for the treatment of more cases. Among the supportive arguments used by proponents of a digital workflow and guided surgery is the improved accuracy in implant positioning. However, it is important to recognize that there is limited data comparing dynamic, computer-navigated surgery and static, template-based surgery with manual, nonguided implant placement.19
In a systematic review by Jung et al,20 in which most of the studies evaluated focused on dynamic systems and template-based systems, the results indicated that reported accuracy in entry point, apex, depth, and angulation could be influenced by the study design and whether it was model, cadaver, or human-based. Despite limited evidence, Jung and colleagues concluded that dynamic systems were more precise than static systems and that accuracy was better in model and cadaver studies than in human studies. The clinical, patient-based studies tended to evaluate intraoperative complications, treatment time, and postoperative implant stability. They found that: (1) guided, immediately restored implant cases resulted in lower implant failure rates, (2) intrasurgical complications included restricted interocclusal distances complicating site preparation and inadequate primary implant stability, (3) fewer complications occurred in flapless versus open flap surgeries (most significantly in the treatment of edentulous patients), and (4) greater pain perception was associated with open flap versus flapless surgery using a visual analog scale. Most of the studies assessed in this review were of a short duration and did not provide long-term data on implant survival following guided placement. A more recent report by Ravidà and colleagues, which compared computer-guided implant placement with traditional nonguided implant placement for implant-supported hybrid prostheses, revealed that the treatment costs for each were comparable, but computer-guided implant placement resulted in higher implant survival rates.21
An International Team for Implantology consensus report on digital technologies concluded that static, computer-aided implant surgery was superior to conventional implant surgery based on patient pain and discomfort reports, economics, and intraoperative complications and that a higher level of accuracy was achieved in partially edentulous versus fully edentulous patients. Furthermore, they indicated that static, computer-aided surgery should be considered as an additional tool for comprehensive diagnosis, treatment planning, and surgical procedures.22
The use of a digital planning workflow ultimately leads to the use of systems that enhance surgical treatment accuracy and predictability through either dynamic or static surgical guidance.23 Dynamic guided surgical systems (ie, real-time drill tracking relative to a planned trajectory on a CBCT scan with a virtual restoration, haptic feedback) have been successfully incorporated into practice by many clinicians. Although these systems require a significant financial investment, they may reduce the per-case cost and allow for expedited treatment through the elimination of lab-produced or dentist-fabricated surgical guides. Some clinicians argue that static guides can potentially introduce more error in implant positioning due to guide instability or movement during treatment. This can be avoided by selecting an appropriate guide design with stabilization and support mechanisms incorporated. When fixation pins are used, complete guide seating and stability (ie, no rocking) on teeth, soft tissues, or bone should be verified through inspection windows or occlusal analysis.24,25 One of the issues that must be considered when investing in a dynamic navigation system is the space requirement for the "footprint" of these units. A primary advantage of dynamic systems is that there is no need for manufacturer-specific guided surgical kits, which allows for the use of a standard implant surgical kit, and another is that they can be used more efficiently in areas with restricted intraoral access.26 For many static systems, the overall length of the handpiece and attached drills can make it difficult to maneuver and insert into a master cylinder.
Considerations for Implementation
Although there are many benefits to adopting a digital practice philosophy and workflow, there can also be barriers to overcome. One such barrier is the learning curve associated with using the instruments and technology. This is a real problem that can be encountered at both the clinician and staff levels, and it requires a properly qualified and experienced trainer. Another issue is identifying a CBCT scanner and intraoral scanner that offer the highest level of technology with adequate reseller support. For clinicians who are venturing into digital implant dentistry, this can be one of the biggest hurdles in implementation, which often results in optical scanners sitting unused simply due to challenges in learning how to scan and negotiate the planning software.
Dedicating time to case planning can also present an obstacle. This relates to the nonclinical steps of merging data sets, designing and articulating the virtual reconstruction using various software programs, and planning the implant positions. Although the time required to complete these steps can be reduced through experience, completing the design and implant planning can be demanding, especially as case complexity increases. Trained support staff can manage some of the steps, but ultimately, the treating clinicians must approve the final plan.
One solution to address these time-re-la-ted issues is to outsource to laboratories or planning facilities. In a sense, working with parties who are "experts" in design and manufacturing is much like referring to specialists in various dental disciplines. In fact, this has been a key to success in streamlining the process of incorporating digital planning and guided surgery so that it is now a routine surgical protocol. Successful collaboration can require taking time to educate the technician on CBCT scan interpretation in order to ideally position the prescribed implants. Even with training, technicians may fail to produce the desired implant plan exactly as prescribed by the clinician. However, plan approval is still the responsibility of the clinician, and errors cannot be blamed on poor decisions made by the planning team. Poorly positioned implants are rarely attributed to the digital technology used. More often, they are attributed to human error, especially during the virtual planning phase. Always ensure that any laboratory or planning facility used has a method of accepting scans and prescriptions that is compliant with the Health Insurance Portability and Accountability Act (HIPAA) privacy and security rules.
When adopting a digital implant workflow, clinicians must decide whether the printing of surgical templates and printing or milling of transitional prosthetic components will be done in-office or outsourced. A recent case series report by Bencharit and colleagues27 that studied the accuracy of desktop-printed guides indicated that using intraoral scanning with CBCT imaging and then virtually planning implant positions with software resulted in greater accuracy when used with fully-guided surgery than with partially-guided surgery. Deviations are possible in all guided surgical procedures, and in this report, their occurrence was influenced by the site and location (eg, posterior implants had greater deviations in planned versus executed positions than anterior implants).
Printers that are relatively inexpensive to purchase can be slow to print, whereas higher-cost printers can facilitate more rapid and large-scale print jobs. This offers total control of all of the steps for the digitally savvy clinician but may be perceived as daunting to the digitally novice clinician. Clinicians must consider whether or not the per-guide reduction in cost and increase in control outweigh the initial financial investment of purchasing a printer and the time investment required to design and finish the generated appliances. In an established and busy practice where limited staffing appears to be the current norm, in-office printing may not be advantageous.
Conclusion
Success in adopting a digital implant work-flow depends on ones' openness to change. Although this can certainly present challenges for the clinician, his or her staff, and the practice, innovation and technology continue to positively shape today's dental treatments. When clinicians recognize that they can collaborate with colleagues and technicians, it helps to facilitate the adoption and use of the required tools and technology. Ultimately, the goal is to enhance the patient experience, improve treatment outcomes, and reduce complications. The benefits for clinicians include stress reduction, increased profitability, and an elevation of the treatment standard that is worth aspiring to. In adopting a digital workflow, clinicians are pushing themselves to do better dentistry, and by doing so, they are setting the example for future generations of clinicians.
About the Author
Sonia Leziy, DDS, Dipl Perio Fellow
The College of Dental Surgeons of British Columbia
Fellow
The Royal College of Dentists of Canada
Private Practice
North Vancouver, British Columbia
References
1. Rios HF, Borgnakke WS, Benavides E. The use of cone-beam computed tomography in management of patients requiring dental implants: an American Academy of Periodontology best evidence review. J Periodontol. 2017;88(10):946-959.
2. Jacobs R, Salmon B, Codari M, et al. Cone beam computed tomography in implant dentistry: recom-mendations for clinical use. BMC Oral Health. 2018;18(1):88.
3. Bohner L, Gamba DD, Hanisch M, et al. Accuracy of digital technologies for the scanning of facial, skeletal, and intraoral tissues: a systematic review. J Prosthet Dent. 2019;121(2):246-251.
4. Rutkūnas V, Gečiauskaitė A, Jegelevičius D, et al. Accuracy of digital implant impressions with intraoral scanners. A systematic review. Eur J Oral Implantol. 2017;10(Suppl 1):101-120.
5. Mangano F, Veronesi G. Digital versus analog procedures for the prosthetic restoration of single implants: a randomized controlled trial with 1 year of follow-up. Biomed Res Int. 2018;2018:5325032. doi:10.1155/2018/5325032.
6. Joda T, Brägger U. Time-efficiency analysis comparing digital and conventional workflows for implant crowns: a prospective clinical crossover trial. Int J Oral Maxillofac Implants. 2015;30(5):1047-1053.
7. Joda T, Lenherr P, Dedem P, et al. Time efficiency, difficulty, and operator's preference comparing digital and conventional implant impressions: a randomized controlled trial. Clin Oral Implants Res. 2017;28
(10):1318-1323.
8. Norkin F, Ganeles J, Zfaz S, et al. Image-guided implant surgery in today's clinical practice. Inside Dentistry. 2013;9(12):44-48.
9. Vandenberghe B. The digital patient - imaging science in dentistry. J Dent. 2018:74(Suppl 1):S21-S26.
10. Gallucci GO, Joda T. The virtual patient in dental medicine. Clin Oral Implants Res. 2015;26(6):725-726.
11. Joda T, Brägger U. Digital vs. conventional implant prosthetic workflows: a cost/time analysis. Clin Oral Implants Res. 2015;26(12):1430-1435.
12. Joda T, Ferrari M, Gallucci GO et al. Digital technology in fixed implant prosthodontics. Periodontol 2000. 2017;73(1):178-192.
13. Zimmermann M, Mehl A. Virtual smile design systems: a current review. Int J Comput Dent. 2015;18(4):303-317.
14. Edelhoff D, Probst F, Ehrenfeld M, et al. Inter-disciplinary full-mouth rehabilitation for redefining esthetics, function, and orofacial harmony. J Esthet Restor Dent. 2019. doi: 10.1111/jerd.12455.
15. D'Souza KM, Aras MA. Types of implant surgical guides in dentistry: a review. J Oral Implantol. 2012;38
(5):643-652.
16. Vermeulen J. The accuracy of implant placement by experienced surgeons: guided vs freehand approach in a simulated plastic model. Int J Oral Maxillofac Implants. 2017;32(3):617-634.
17. Arunyanak SP, Harris BT, Grant GT, et al. Digital approach to planning computer-guided surgery and immediate provisionalization in a partially edentulous patient. J Prosthet Dent. 2016;116(1):8-14.
18. Tahayeri A, Morgan M, Fugolin AP, et al. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent Mater. 2018;34(2):192-200.
19. Ruppin J, Popovic A, Strauss M, et al. Evaluation of the accuracy of three different computer-aided surgery systems in dental implantology: optical tracking vs. stereolithographic splint systems. Clin Oral Implants Res. 2008;19(7):709-716.
20. Jung RE, Schneider D, Ganeles J, et al. Computer technology applications in surgical implant dentistry: a systematic review. Int J Oral Maxillofac Implants. 2009;24 Suppl: 92-109.
21. Ravidà A, Barootchi S, Tattan M, et al. Clinical outcomes and cost effectiveness of computer-guided versus conventional implant-retained hybrid prostheses: a long-term retrospective analysis of treatment protocols. J Periodontol. 2018;89(9):1015-1024.
22. Wismeijer D, Joda T, Flügge T, et al. Group 5 ITI consensus report: digital technologies. Clin Oral Implants Res. 2018;29 Suppl 16:436-442.
23. Gulati M, Anand V, Salaria SK, et al. Computerized implant-dentistry: advances toward automation. J Indian Soc Periodontol. 2015;19(1):5-10.
24. Sigcho López DA, García I, Da Silva Salomao G, et al. Potential deviation factors affecting stereolithographic surgical guides: A systematic review. Implant Dent. 2019;28(1):68-73.
25. D'haese J, Ackhurst J, Wismeijer D, et al. Current state of the art of computer-guided implant surgery. Periodontol 2000. 2017;73(1):121-133.
26. Emery RW, Merritt SA, Lank K, et al. Accuracy of dynamic navigation for dental implant placement-model-based evaluation. J Oral Implantol. 2016;42(5):399-405.
27. Bencharit S, Staffen A, Yeung M, et al. In vivo tooth-supported implant surgical guides fabricated with desktop stereolithographic printers: fully guided surgery is more accurate than partially guided surgery. J Oral Maxillofac Surg. 2018;76(7):1431-1439.