A Contemporary Endodontic Approach
New class of silicate-based cements offer enhanced biocompatibility
Leandro A. P. Pereira, DDS, MSc, PhD
With the evolution of intracanal microbiological knowledge and the impact of new canal modeling instruments with continuous or alternating rotation, we now know that it is not possible to completely eliminate the microorganisms inside the endodontic microanatomy. However, we also know that this is not necessary for success and that a significant reduction in the levels of intracanal infection, in most cases, is sufficient.1 This can be accomplished by creating an intercanal environment at the time of obturation that is unfavorable to the population growth of the remaining bacteria. Therefore, another function of obturation is to prevent or hinder the growth of residual bacteria not eliminated during the cleaning and disinfection process.
To be used clinically and achieve the desired objectives, obturation cements must have certain essential properties, including having the capacity to fill, seal, and present dimensional stability; not being soluble in the organic tissue fluids; having a film thickness of no more than 50 micrometers; being radiopaque; having good drainage; not producing chromatic alterations; having suitable working time; being easy to set, manipulate, and remove if necessary; promoting cementogenesis; and being biocompatible and non-irritating to the tissues of the periapex.2
With the advancement of new materials and rehabilitative concepts in the era of adhesive dentistry, the pursuit of two other characteristics has become increasingly important in the development of new endodontic cements. The first is the absence of eugenol, which interferes with the strength of the bond in resin systems.3 The second characteristic is bioactivity. Bioactivity is the capacity of a material to be integrated with the tissues and structures of an organism with which it is in contact.
The bioactivity of mineral trioxide aggregate (MTA) is known as biomineralization and was first described by Reyes and Carmona in 2009. In one "in vitro" study, the authors used scanning electron microscopy to observe the integration of the MTA with the dentin through deposition of numerous apatite groups on the dental collagen fibrils throughout the dentinal tubule surface in contact with the MTA. Interestingly, the authors observed that the more contact time the material had with the dentin, the more extensive the mineralizations were. These mineralizations may be responsible for the superior adaptation of this material to the dentin.4,5
Unfortunately, the low drainage capacity of MTA does not allow for its use as an obturating cement. Thus, to get the benefit of this material's biocompatibility, a new class of obturating endodontic cement was created: silicate-based cements. This designation is derived from the components that are present in these cements, including tricalcium silicate, dicalcium silicate, calcium oxide, and tricalcium aluminate. The MTA Fillapex is a resin-based endodontic sealer that contains amounts of MTA.
The clinical case below highlights the use of Fillapex MTA cement (Angelus) along with gutta-percha cones for an endodontic obturation treatment performed in a single session.
A 56-year-old, female Caucasian patient presented to the office complaining of spontaneous, pulsing pain in the left mandible region, which did not cease with the use of analgesics and anti-inflammatories. She had a negative response to the tests of apical palpation and vertical and lateral percussion on all of the teeth in this quadrant, and thermal tests showed an exacerbated, long-duration positive response to both cold and heat on tooth No. 37. Regarding the other teeth in the quadrant, a slight, short-duration positive response was shown to cold, with a negative response to heat.
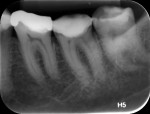
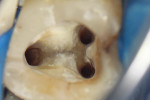
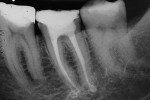
According to the classification system of the American Endodontics Association, tooth No. 36 had a pulpal and periapical diagnosis of irreversible inflammatory pulpitis with normal periapex, for which endodontic treatment is indicated (Figure 1 through Figure 3).6
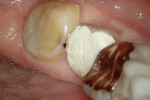
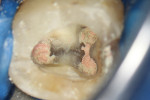
The treatment was conducted in its entirety using an operative microscope, with magnification varying between 2.5X and 12.5X. The pulp chamber was accessed with a 1013 spherical diamond bit followed by a 3082 conical-truck diamond bit (Figure 4), and the finishing was done with a conical-truck diamond ultrasonic tip (Helse, E7D). After location of the canals, a No. 10 type K file was slowly introduced until reaching two-thirds of the initial x-ray length of the tooth. This was followed by a No. 25.06 reciprocating instrument (Reciproc, VDW) with apical progression in sequences of three movements around 1mm in amplitude in the apical direction. Following each sequence of three movements with the reciprocating instrument, irrigation was performed with 5ml of 2.5% sodium hypochlorite, and a No. 10 type K file was taken to two-thirds of the x-ray length of the tooth. This procedure was repeated until the reciprocating instruments reached this pre-established length.
The next step was to conduct electronic odontometry with a foramen locator and establish the real work length. Subsequently, the diameter of the region was verified through the introduction of different calibers of manual type K files until one of them was observed to adapt to the lateral walls of the canals. In the mesial canals, the instrument that adapted to this region was the No. 30, and in the distal canal, the No. 40. In this way, and using the same initial operative sequence (ie preparation, modeling, and irrigation), the mesial canals were prepared for the Reciproc 40 VDWinstrument and the distal canals were prepared for the Reciproc 50 VDW instrument (Figure 5).
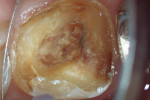
After modeling, the system of canals was dried and filled with 17% EDTA-T, and an Irrisonic ultrasound tip (Helse) was used to passively activate the substance for three cycles of 15 seconds with renewal of the substance for each cycle. After the passive activation through ultrasound, the canals were again irrigated with 5ml of 2.5% sodium hypochlorite, and the main gutta-percha cones were tested and adjusted. Finally, the system of canals was dried with aspiration micro-cannulas connected to a vacuum suctor.
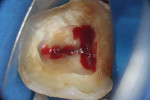
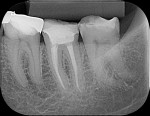
To complete the procedure, the Fillapex MTA cement was prepared and introduced into the canals using the main gutta-percha cones (Figure 6). The excess material from the cones was cut using a heat transfer system (Touch'n Heat Sybron Endo) and cold-compressed vertically. The pulp chamber was sealed with photopolymerizable composite resin, and the patient was sent to her dentist for a definitive restoration of the dental element (Figure 7). After 17 months, the patient returned for a control consultation, and an evaluation of her x-ray images indicated endodontic success characterized by the absence of signs and symptoms, the physiologic function of the tooth, the normality of the periapex, and the reabsorption of the surplus Fillapex MTA cement (Figure 8).
References
1. Siqueira JF Jr., Rôças, IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34(11):1291-1301.
2. Hargreaves KM, Cohen S, Berman LH. Cohen's Pathways of the Pulp. 10th ed. Mosby Elsevier; 2011.
3. Vano M, Cury AH, Goracci C, Chieffi N, Gabriele M, Tay FR, Ferrari M. The effect of immediate versus delayed cementation on the retention of different types of fiber post in canals obturated using a eugenol sealer. J Endod. 2006;32(9):882-885.
4. Reyes-Carmona JF, Felippe MS, Felippe WT. BioÂmineralization ability and interaction of mineral trioxide aggregate and white portland cement with dentin in a phosphate-containing fluid. J Endod. 2009;35(5):731-736.
5. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21(7):349-353.
6. Glickman GN. AAE consensus conference on diagnostic terminology: background and perspectives. J Endod. 2009;35(12):1619-1620.
For more information, contact:
Angelus
855-346-3682
www.angelusdental.com